Abstract
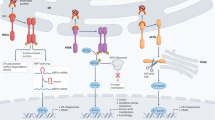
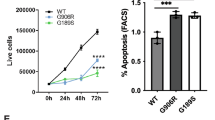
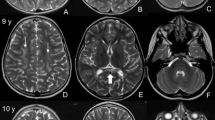
Krabbe disease, one of the autosomal-recessive lysosomal storage disorders (LSDs), is caused by a deficiency of galactocerebrosidase (GALC) activity, resulting in the intracellular accumulation of psychosine, which is cytotoxic for neuronal cells. Genetically pathogenic mutations result in conformational changes in GALC and disrupt the lysosmal trafficking of cargos, which subsequently accumulate in the endoplasmic reticulum (ER). Recently, ER stress together with the activation of the unfolded protein response (UPR) has been suggested to play a key role in the pathogenesis of LSDs. In this study, we hence investigated whether the UPR is activated in Krabbe disease using COS-7 cells expressing pathogenic GALC mutants and skin fibroblasts (SFs) from Krabbe disease patients with various phenotypes, using a combination of semiquantitative and quantitative real-time polymerase chain reactions. We found that UPR activation in Krabbe disease depends on the mutations and cell types, and there is the possibility that multiple pathways, involving ER chaperones, inositol-requiring kinase 1, and protein kinase regulated by RNA-like ER kinase are activated by mutations associated with the infantile form. These results indicate that in Krabbe disease, each misfolded/unfolded protein evokes different UPR activation depending on the mutation, and that the activated pathways affect the phenotypes.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Suzuki K, Suzuki Y. Globoid cell leucodystrophy (Krabbe’s disease): deficiency of galactocerebroside β-galactosidase. Proc Natl Acad Sci USA. 1970;66:302–9.
Chen YQ, Rafi MA, de Gala G, Wenger DA. Cloning and expression of cDNA encoding human galactocerebrosidase, the enzyme deficient in globoid cell leukodystrophy. Hum Mol Genet. 1993;2:1841–5.
Sakai N, Inui K, Fujii N, Fukushima H, Nishimoto J, Yanagihara I, et al. Krabbe disease: isolation and characterization of a full-length cDNA for human galactocerebrosidase. Biochem Biophys Res Commun. 1994;198:485–91.
Nagano S, Yamada T, Shinnoh N, Furuya H, Taniwaki T, Kira J. Expression and processing of recombinant human galactosylceramidase. Clin Chim Acta. 1998;276:53–61.
Hossain MA, Otomo T, Saito S, Ohno K, Sakuraba H, Hamada Y, et al. Late-onset Krabbe disease is predominant in Japan and its mutant precursor protein undergoes more effective processing than the infantile-onset form. Gene. 2014;534:144–54.
Lee WC, Kang D, Causevic E, Herdt AR, Eckman EA, Eckman CB. Molecular characterization of mutations that cause globoid cell leukodystrophy and pharmacological rescue using small molecule chemical chaperones. J Neurosci. 2010;30:5489–97.
Spratley SJ, Hill CH, Viuff AH, Edgar JR, Skjødt K, Deane JE. Molecular mechanisms of disease pathogenesis differ in Krabbe disease variants. Traffic. 2016;17:908–22.
Nagara H, Ogawa H, Sato Y, Kobayashi T, Suzuki K. The twitcher mouse: degeneration of oligodendrocytes in vitro. Brain Res. 1986;391:79–84.
Tanaka K, Nagara H, Kobayashi T, Goto I. The twitcher mouse: accumulation of galactosylsphingosine and pathology of the sciatic nerve. Brain Res. 1988;454:340–6.
Suzuki K. Twenty five years of the “psychosine hypothesis”: a personal perspective of its history and present status. Neurochem Res. 1998;23:251–9.
Kaufman RJ. Stress signaling from the lumen of the endoplasmic reticulum: coordination of gene transcriptional and translational controls. Genes Dev. 1999;13:1211–33.
Rutkowski DT, Kaufman RJ. A trip to the ER: coping with stress. Trends Cell Biol. 2004;14:20–8.
Schröder M, Kaufman RJ. The mammalian unfolded protein response. Annu Rev Biochem. 2005;74:739–89.
Shi Y, Vattem KM, Sood R, An J, Liang J, Stramm L, Wek RC. Identification and characterization of pancreatic eukaryotic initiation factor 2 α-subunit kinase, PEK, involved in translational control. Mol Cell Biol. 1998;18:7499–509.
Harding HP, Zhang Y, Ron D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature. 1999;397:271–4.
Wei H, Kim SJ, Zhang Z, Tsai PC, Wisniewski KE, Mukherjee AB. ER and oxidative stresses are common mediators of apoptosis in both neurodegenerative and non-neurodegenerative lysosomal storage disorders and are alleviated by chemical chaperones. Hum Mol Genet. 2008;17:469–77.
Tessitore A, del P Martin M, Sano R, Ma Y, Mann L, Ingrassia A, et al. GM1-ganglioside-mediated activation of the unfolded protein response causes neuronal death in a neurodegenerative gangliosidosis. Mol Cell. 2004;15:753–66.
Zhang Z, Lee YC, Kim SJ, Choi MS, Tsai PC, Xu Y, et al. Palmitoyl-protein thioesterase-1 deficiency mediates the activation of the unfolded protein response and neuronal apoptosis in INCL. Hum Mol Genet. 2006;15:337–46.
Villani GR, Chierchia A, Di Napoli D, Di Natale P. Unfolded protein response is not activated in the mucopolysaccharidoses but protein disulfide isomerase 5 is deregulated. J Inherit Metab Dis. 2012;35:479–93.
Suzuki T, Shimoda M, Ito K, Hanai S, Aizawa H, Kato T, et al. Expression of human Gaucher disease gene GBA generates neurodevelopmental defects and ER stress in Drosophila eye. PLoS ONE. 2006;8:e69147 https://doi.org/10.1371/journal.pone.0069147
Sakai N, Fukushima H, Inui K, Fu L, Nishigaki T, Yanagihara I, et al. Human galactocerebrosidase gene: promoter analysis of the 5’-flanking region and structural organization. Biochim Biophys Acta. 1998;1395:62–7.
Wenger DA, Rafi MA, Luzi P, Datto J, Costantino-Ceccarini E. Krabbe disease: genetic aspects and progress toward therapy. Mol Genet Metab. 2000;70:1–9.
Xu C, Sakai N, Taniike M, Inui K, Ozono K. Six novel mutations detected in the GALC gene in 17 Japanese patients with Krabbe disease, and new genotype-phenotype correlation. J Hum Genet. 2006;51:548–54.
Wiederschain G, Raghavan S, Kolodny E. Characterization of 6-hexadecanoylamino4-methylumbelliferyl-β-D-galactopyranoside as fluorogenic substrate of galactocerebrocidase for the diagnosis of Krabbe disease. Clin. Chim. Acta. 1992;205:87–96.
Zhong W, Chen X, Jiang P, Wan JM, Qin P, Yu AC. Induction of endoplasmic reticulum stress by sonoporation: linkage to mitochondria-mediated apoptosis initiation. Ultrasound Med Biol. 2013;39:2382–92.
Shin D, Feltri ML, Wrabetz L. Altered Trafficking and processing of GALC mutants correlates with globoid cell leukodystrophy severity. J Neurosci. 2016;10:1858–70.
Hossain MA, Higaki K, Saito S, Ohno K, Sakuraba H, Nanba E, et al. Chaperon therapy for Krabbe disease: potential for late-onset GALC mutations. J Hum Genet. 2015;60:539–45.
Lee WC, Tsoi YK, Dickey CA, Delucia MW, Dickson DW, Eckman CB. Suppression of galactosylceramidase (GALC) expression in the twitcher mouse model of globoid cell leukodystrophy (GLD) is caused by nonsense-mediated mRNA decay (NMD). Neurobiol Dis. 2006;23:273–80.
Becker FT, Vitner E, Dekel H, Leshem N, Enquist BI, Karlsson S, et al. No evidence for activation of the unfolded protein response in neuronopathic models of Gaucher disease. Hum Mol Genet. 2009;18:1482–88.
Kondo S, Murakami T, Tatsumi K, Ogata M, Kanemoto S, Otori K, et al. OASIS, a CREB/ATF-family member, modulates UPR signalling in astrocytes. Nat Cell Biol. 2005;7:186–94.
Kim DY, Kim HR, Kim KK, Park JW, Lee BJ. NELL2 function in the protection of cells against endoplasmic reticulum stress. Mol Cells. 2015;38:145–50.
Daffu G, Sohi J, Kamholz J. Proteolipid protein dimerization at cysteine 108: Implications for protein structure. Neurosci Res. 2012;74:144–55.
Acknowledgements
We thank the patients and their families for their participation in this study. We thank K. Tsujimoto (Osaka University) for technical assistance. This work was supported by the Practical Research Project for Rare/Intractable Diseases (15ek0109151h003) from Japan Agency for Medical Research and Development (AMED), and a Health and Labour Sciences Research Grants (H29-nanchitou (nan)-ippan-024) from Ministry of Health, Labour and Welfare.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Irahara-Miyana, K., Otomo, T., Kondo, H. et al. Unfolded protein response is activated in Krabbe disease in a manner dependent on the mutation type. J Hum Genet 63, 699–706 (2018). https://doi.org/10.1038/s10038-018-0445-8
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s10038-018-0445-8
This article is cited by
-
A scoping review of stem cell models of leukodystrophies: advances in understanding pathophysiological mechanisms
npj Genomic Medicine (2025)
-
Identifying altered developmental pathways in human globoid cell leukodystrophy iPSCs-derived NSCs using transcriptome profiling
BMC Genomics (2023)
-
Sphingolipid lysosomal storage diseases: from bench to bedside
Lipids in Health and Disease (2021)
-
A Novel Homozygous Missense Variant in the NAGA Gene with Extreme Intrafamilial Phenotypic Heterogeneity
Journal of Molecular Neuroscience (2020)