Abstract
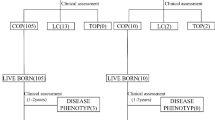
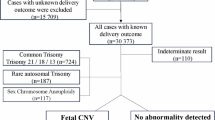
Noninvasive prenatal testing diagnoses fetal aneuploidies in singleton pregnancies with trisomy 21, 18, or 13 accurately. However, clinical data on noninvasive prenatal testing in women with twin or vanishing twin pregnancies are limited. We report on the accuracy and prenatal and neonatal outcomes of noninvasive prenatal testing in twin and vanishing twin pregnancies. This retrospective study was conducted at 22 facilities belonging to the Noninvasive Prenatal Testing Consortium, part of a nationwide project in Japan, visited by women with twin or vanishing twin pregnancies between January 2015 and March 2022. This study investigated the accuracy and perinatal and neonatal outcomes of noninvasive prenatal testing using massively parallel sequencing in twin or vanishing twin pregnancies. Of 1013 women with twin pregnancies, 986 (97.3%) had negative; 13 (1.3%), positive; and 14 (1.4%), non-reportable noninvasive prenatal testing results. Of 225 women with vanishing twins, 203 (90.2%) had negative; 11 (4.9%), positive; and 11 (4.9%), non-reportable results. Among 1693 fetuses (77.3%) excluding 497 unknowns were available for follow-up, 1476 were from twin pregnancies, and 134 were from vanishing twin pregnancies, totaling 1610 babies has born. No false negatives were observed in the cases followed. These results indicate that noninvasive prenatal testing is useful for vanishing twin pregnancies and provide reassurance for pregnant women with twins and vanishing twins.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Chiu RWK, Chan KCA, Gao Y, Lau VYM, Zheng W, Leung TY, et al. Noninvasive prenatal diagnosis of fetal chromosomal aneuploidy by massively parallel genomic sequencing of DNA in maternal plasma. Proc Natl Acad Sci USA. 2008;105:20458–63.
Samura O, Sekizawa A, Suzumori N, Sasaki A, Wada S, Hamanoue H, et al. Current status of non-invasive prenatal testing in Japan. J Obstet Gynaecol Res. 2017;43:1245–55.
Sago H, Sekizawa A, Japan NIPT consortium. Nationwide demonstration project of next-generation sequencing of cell-free DNA in maternal plasma in Japan: 1-year experience. Prenat Diagn. 2015;35:331–6.
Palomaki GE, Deciu C, Kloza EM, Lambert-Messerlian GM, Haddow JE, Neveux LM, et al. DNA sequencing of maternal plasma reliably identifies trisomy 18 and trisomy 13 as well as Down syndrome: an international collaborative study. Genet Med. 2012;14:296–305.
Yotsumoto J, Sekizawa A, Suzumori N, Yamada T, Samura O, Nishiyama M, et al. A survey on awareness of genetic counseling for non-invasive prenatal testing: the first year experience in Japan. J Hum Genet. 2016;61:995–1001.
Leung TY, Qu JZZ, Liao GJW, Jiang P, Cheng YKY, Chan KCA, et al. Noninvasive twin zygosity assessment and aneuploidy detection by maternal plasma DNA sequencing. Prenat Diagn. 2013;33:675–81.
Palomaki GE, Chiu RWK, Pertile MD, Sistermans EA, Yaron Y, Vermeesch JR, et al. International society for prenatal diagnosis position statement: cell free (cf)DNA screening for Down syndrome in multiple pregnancies. Prenat Diagn. 2021;41:1222–32.
Dungan JS, Klugman S, Darilek S, Malinowski J, Akkari YMN, Monaghan KG, et al. Noninvasive prenatal screening (NIPS) for fetal chromosome abnormalities in a general-risk population: an evidence-based clinical guideline of the American College of Medical Genetics and Genomics (ACMG). Genet Med. 2023;25:100336.
National Health Service. Screening for Down’s syndrome, Edwards’ syndrome and Patau’s syndrome: NIPT. Updated 8 October 2024 https://www.gov.uk/government/publications/screening-for-downs-syndrome-edwards-syndrome-and-pataus-syndrome-non-invasive-prenatal-testing-nipt/screening-for-downs-syndrome-edwards-syndrome-and-pataus-syndrome-nipt. Accessed March 14, 2025.
American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins—Obstetrics; Committee on Genetics; Society for Maternal-Fetal Medicine. Screening for fetal chromosomal abnormalities: ACOG practice bulletin, number 226. Obstet Gynecol. 2020;136:e48–69.
van Eekhout JCA, Bekker MN, Bax CJ, Galjaard RJH. Non-invasive prenatal testing (NIPT) in twin pregnancies affected by early single fetal demise: a systematic review of NIPT and vanishing twins. Prenat Diagn. 2023;43:829–37.
He Y, Wang Y, Li Z, Chen H, Deng J, Huang H, et al. Clinical performance of non-invasive prenatal testing for trisomies 21, 18 and 13 in twin pregnancies: a cohort study and a systematic meta-analysis. Acta Obstet Gynecol Scand. 2020;99:731–43.
Takeda E, Suzumori N, Kumagai K, Inuzuka S, Oseto K, Ohigashi Y, et al. Performance and outcomes of noninvasive prenatal testing for twin pregnancies in Japan. J Obstet Gynaecol Res. 2018;44:1909–14.
Boo HY, Han YJ. Cell-free DNA screening in twin pregnancies. Obstet Gynecol Sci. 2024;67:160–8.
Khalil A, Archer R, Hutchinson V, Mousa HA, Johnstone ED, Cameron MJ, et al. Noninvasive prenatal screening in twin pregnancies with cell-free DNA using the IONA test: a prospective multicenter study. Am J Obstet Gynecol. 2021;225:79.e1–79.e13.
Vink J, Wapner R, D’Alton ME. Prenatal diagnosis in twin gestations. Semin Perinatol. 2012;36:169–74.
Konishi A, Samura O, Muromoto J, Okamoto Y, Takahashi H, Kasai Y, et al. Prevalence of common aneuploidy in twin pregnancies. J Hum Genet. 2022;67:261–5.
Kantor V, Mo L, DiNonno W, Howard K, Palsuledesai CC, Parmar S, et al. Positive predictive value of a single nucleotide polymorphism (SNP)-based NIPT for aneuploidy in twins: Experience from clinical practice. Prenat Diagn. 2022;42:1587–93.
Grömminger S, Yagmur E, Erkan S, Nagy S, Schöck U, Bonnet J, et al. Fetal Aneuploidy Detection by Cell-Free DNA Sequencing for Multiple Pregnancies and Quality Issues with Vanishing Twins. J Clin Med. 2014;25:679–92.
Dobson LJ, Reiff ES, Little SE, Wilkins-Haug L, Bromley B. Patient choice and clinical outcomes following positive noninvasive prenatal screening for aneuploidy with cell-free DNA (cfDNA). Prenat Diagn. 2016;36:456–62.
Health Science Council’s Subcommittee for the Evaluation of Advanced Medical Technology and Expert Committee on Assisted Reproductive Technology. Report on Assisted Reproductive Medicine by Donation of Sperm, Egg, Embryo. 2000. https://www.mhlw.go.jp/www1/shingi/s0012/s1228-1_18.html#3. Accessed August 5, 2024.
Acknowledgements
We wish to thank the women who participated in this study and members of the NIPT Consortium for facilitating the nationwide project in Japan.
Funding
This study was supported by Japan Society for the Promotion of Science, Grant-in-Aid for Early-Career Scientists (grant number: 24K20343).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Takeda, E., Suzumori, N., Samura, O. et al. Significance of noninvasive prenatal testing using massively parallel sequencing in women with twin or vanishing twin pregnancies. J Hum Genet 70, 297–305 (2025). https://doi.org/10.1038/s10038-025-01332-2
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s10038-025-01332-2