Abstract
Chondroitin sulfate (CS)/dermatan sulfate (DS) proteoglycans that play indispensable roles in multiple physiological processes, including cell proliferation, cell adhesion, development, neuronal guidance, and cartilage formation. Depletion of CS/DS caused by biosynthetic enzyme loss of function impairs these processes and results in embryonic lethality. However, some individuals with mutant enzymes survive and exhibit severe phenotypes. These rare hereditary diseases have been discovered and characterized in recent decades because of marked advances in next-generation sequencing technology. In this review, CS/DS-related inherited diseases caused by aberrations in both CS/DS backbone synthesis, as well as their sulfation and/or epimerization, are comprehensively summarized and their pathogenesis discussed.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Couchman JR. Transmembrane signaling proteoglycans. Annu Rev Cell Dev Biol. 2010;26:89–114.
Kim SH, Turnbull J, Guimond S. Extracellular matrix and cell signalling: the dynamic cooperation of integrin, proteoglycan, and growth factor receptors. J Endocrinol. 2011;209:139–51.
Mizumoto S, Yamada S, Sugahara K. Molecular interactions between chondroitin-dermatan sulfate and growth factors/receptors/matrix proteins. Curr Opin Struct Biol. 2015;34:35–42.
Hayashida K, Aquino RS, Park PW. Coreceptor functions of cell surface heparan sulfate proteoglycans. Am J Physiol Cell Physiol. 2022;322:C896–C912.
Mitsou I, Multhaupt HAB, Couchman JR. Proteoglycans, ion channels and cell-matrix adhesion. Biochem J. 2017;474:1965–79.
Gómez Toledo A, Sorrentino JT, Sandoval DR, Malmström J, Lewis NE, Esko JD. A Systems View of the Heparan Sulfate Interactome. J Histochem Cytochem. 2021;69:105–19.
Mizumoto S, Ikegawa S, Sugahara K. Human genetic disorders caused by mutations in genes encoding biosynthetic enzymes for sulfated glycosaminoglycans. J Biol Chem. 2013;288:10953–61.
Mizumoto S, Yamada S, Sugahara K. Mutations in biosynthetic enzymes for the protein linker region of chondroitin/dermatan/heparan sulfate cause skeletal and skin dysplasias. Biomed Res Int. 2015;2015:861752.
Mizumoto S, Yamada S. Congenital disorders of deficiency in glycosaminoglycan biosynthesis. Front Genet. 2021;12:717535.
Xie C, Schaefer L, Iozzo RV. Global impact of proteoglycan science on human diseases. iScience. 2023;26:108095.
Kobayashi H. Recent trends in mucopolysaccharidosis research. J Hum Genet. 2019;64:127–37.
Howie AH, Tingley K, Inbar-Feigenberg M, Mitchell JJ, Angel K, Gentle J, et al. INFORM RARE Network. Review of clinical trials and guidelines for children and youth with mucopolysaccharidosis: outcome selection and measurement. Orphanet J Rare Dis. 2024;19:393.
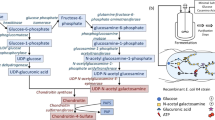
Mikami T, Kitagawa H. Biosynthesis and function of chondroitin sulfate. Biochim Biophys Acta. 2013;1830:4719–33.
Sugahara K, Kitagawa H. Heparin and heparan sulfate biosynthesis. IUBMB Life. 2002;54:163–75.
Koike T, Izumikawa T, Tamura J, Kitagawa H. FAM20B is a kinase that phosphorylates xylose in the glycosaminoglycan-protein linkage region. Biochem J. 2009;421:157–62.
Tone Y, Pedersen LC, Yamamoto T, Izumikawa T, Kitagawa H, Nishihara J, et al. 2-O-phosphorylation of xylose and 6-O-sulfation of galactose in the protein linkage region of glycosaminoglycans influence the glucuronyltransferase-I activity involved in the linkage region synthesis. J Biol Chem. 2008;283:16801–7.
Wen J, Xiao J, Rahdar M, Choudhury BP, Cui J, Taylor GS, et al. Xylose phosphorylation functions as a molecular switch to regulate proteoglycan biosynthesis. Proc Natl Acad Sci USA. 2014;111:15723–8.
Izumikawa T, Sato B, Mikami T, Tamura J, Igarashi M, Kitagawa H. GlcUAβ1-3Gal β1-3Gal β1-4Xyl(2-O-phosphate) is the preferred substrate for chondroitin N-acetylgalactosaminyltransferase-1. J Biol Chem. 2015;290:5438–48.
Koike T, Izumikawa T, Sato B, Kitagawa H. Identification of phosphatase that dephosphorylates xylose in the glycosaminoglycan-protein linkage region of proteoglycans. J Biol Chem. 2014;289:6695–708.
Koike T, Mikami T, Tamura JI, Kitagawa H. Altered sulfation status of FAM20C-dependent chondroitin sulfate is associated with osteosclerotic bone dysplasia. Nat Commun. 2022;13:7952.
Malmström A, Bartolini B, Thelin MA, Pacheco B, Maccarana M. Iduronic acid in chondroitin/dermatan sulfate: biosynthesis and biological function. J Histochem Cytochem. 2012;60:916–25.
Malmström A. Biosynthesis of dermatan sulfate. II. Substrate specificity of the C-5 uronosyl epimerase. J Biol Chem. 1984;259:161–5.
Nakajima M, Mizumoto S, Miyake N, Kogawa R, Iida A, Ito H, et al. Mutations in B3GALT6, which encodes a glycosaminoglycan linker region enzyme, cause a spectrum of skeletal and connective tissue disorders. Am J Hum Genet. 2013;92:927–34.
Bui C, Huber C, Tuysuz B, Alanay Y, Bole-Feysot C, Leroy JG, et al. XYLT1 mutations in Desbuquois dysplasia type 2. Am J Hum Genet. 2014;94:405–14.
Munns CF, Fahiminiya S, Poudel N, Munteanu MC, Majewski J, Sillence DO, et al. Homozygosity for frameshift mutations in XYLT2 result in a spondylo-ocular syndrome with bone fragility, cataracts, and hearing defects. Am J Hum Genet. 2015;96:971–8.
Jamsheer A, Olech EM, Kozłowski K, Niedziela M, Sowińska-Seidler A, Obara-Moszyńska M, et al. Exome sequencing reveals two novel compound heterozygous XYLT1 mutations in a Polish patient with Desbuquois dysplasia type 2 and growth hormone deficiency. J Hum Genet. 2016;61:577–83.
Taylan F, Costantini A, Coles N, Pekkinen M, Héon E, Şıklar Z, et al. Spondyloocular Syndrome: Novel Mutations in XYLT2 Gene and Expansion of the Phenotypic Spectrum. J Bone Miner Res. 2016;31:1577–85.
Silveira C, Leal GF, Cavalcanti DP. Desbuquois dysplasia type II in a patient with a homozygous mutation in XYLT1 and new unusual findings. Am J Med Genet A. 2016;170:3043–47.
Al-Jezawi NK, Ali BR, Al-Gazali L. Endoplasmic reticulum retention of xylosyltransferase 1 (XYLT1) mutants underlying Desbuquois dysplasia type II. Am J Med Genet A. 2017;173:1773–81.
Taylan F, Yavaş Abalı Z, Jäntti N, Güneş N, Darendeliler F, Baş F, et al. Two novel mutations in XYLT2 cause spondyloocular syndrome. Am J Med Genet A. 2017;173:3195–200.
Umair M, Eckstein G, Rudolph G, Strom T, Graf E, Hendig D, et al. Homozygous XYLT2 variants as a cause of spondyloocular syndrome. Clin Genet. 2018;93:913–8.
LaCroix AJ, Stabley D, Sahraoui R, Adam MP, Mehaffey M, Kernan K, et al. University of Washington Center for Mendelian Genomics; Mefford HC, Sol-Church K. GGC Repeat Expansion and Exon 1 Methylation of XYLT1 Is a Common Pathogenic Variant in Baratela-Scott Syndrome. Am J Hum Genet. 2019;104:35–44.
Kausar M, Chew EGY, Ullah H, Anees M, Khor CC, Foo JN, et al. A novel homozygous frameshift variant in XYLT2 causes spondyloocular syndrome in a consanguineous Pakistani family. Front Genet. 2019;10:144.
Götting C, Kuhn J, Kleesiek K. Human xylosyltransferases in health and disease. Cell Mol Life Sci. 2007;64:1498–517.
Ritelli M, Chiarelli N, Zoppi N, Dordoni C, Quinzani S, Traversa M, et al. Insights in the etiopathology of galactosyltransferase II (GalT-II) deficiency from transcriptome-wide expression profiling of skin fibroblasts of two sisters with compound heterozygosity for two novel B3GALT6 mutations. Mol Genet Metab Rep. 2014;2:1–15.
Vorster AA, Beighton P, Ramesar RS. Spondyloepimetaphyseal dysplasia with joint laxity (Beighton type); mutation analysis in eight affected South African families. Clin Genet. 2015;87:492–5.
Salter CG, Davies JH, Moon RJ, Fairhurst J, Bunyan D, DDD Study, Foulds N. Further defining the phenotypic spectrum of B4GALT7 mutations. Am J Med Genet A. 2016;170:1556–63.
Ben-Mahmoud A, Ben-Salem S, Al-Sorkhy M, John A, Ali BR, Al-Gazali L. A B3GALT6 variant in patient originally described as Al-Gazali syndrome and implicating the endoplasmic reticulum quality control in the mechanism of some beta3GalT6-pathy mutations. Clin Genet. 2018;93:1148–58.
Mihalic Mosher T, Zygmunt DA, Koboldt DC, Kelly BJ, Johnson LR, McKenna DS, et al. Expansion of B4GALT7 linkeropathy phenotype to include perinatal lethal skeletal dysplasia. Eur J Hum Genet. 2019;27:1569–77.
Caraffi SG, Maini I, Ivanovski I, Pollazzon M, Giangiobbe S, Valli M, et al. Severe peripheral joint laxity is a distinctive clinical feature of spondylodysplastic-Ehlers-Danlos syndrome (EDS)-B4GALT7 and spondylodysplastic-EDS-B3GALT6. Genes. 2019;10:799.
Lorenz D, Kress W, Zaum AK, Speer CP, Hebestreit H. Report of two siblings with spondylodysplastic Ehlers-Danlos syndrome and B4GALT7 deficiency. BMC Pediatr. 2021;21:293.
Leoni C, Tedesco M, Radio FC, Chillemi G, Leone A, Bruselles A, et al. Broadening the phenotypic spectrum of Beta3GalT6-associated phenotypes. Am J Med Genet A. 2021;185:3153–60.
Shen F, Yang Y, Zheng Y, Tu M, Zhao L, Luo Z, et al. Mutant B3GALT6 in a multiplex family: A dominant variant co-segregated with moderate malformations. Front Genet. 2022;13:824445.
Coetzer KC, Dieckerhoff J, Wollnik B, Moosa S. B3GALT6-linkeropathy: Three illustrative patients spanning the disease spectrum. Eur J Med Genet. 2023;66:104829.
Alessandri JL, Celse T, Spodenkiewicz M, Calaya A, Dumont C, Jacquemont ML, et al. Prenatal and neonatal phenotype of Larsen of La Reunion Island syndrome (B4GALT7-linkeropathy). Eur J Med Genet. 2024;69:104940.
Delbaere S, De Clercq A, Mizumoto S, Noborn F, Bek JW, Alluyn L, et al. b3galt6 knock-out zebrafish recapitulate β3GalT6-deficiency disorders in human and reveal a trisaccharide proteoglycan linkage region. Front Cell Dev Biol. 2020;8:597857.
Persson A, Nilsson J, Vorontsov E, Noborn F, Larson G. Identification of a non-canonical chondroitin sulfate linkage region trisaccharide. Glycobiology. 2019;29:366–71.
Izumikawa T, Kanagawa N, Watamoto Y, Okada M, Saeki M, Sakano M, et al. Impairment of embryonic cell division and glycosaminoglycan biosynthesis in glucuronyltransferase-I-deficient mice. J Biol Chem. 2010;285:12190–6.
Baasanjav S, Al-Gazali L, Hashiguchi T, Mizumoto S, Fischer B, Horn D, et al. Faulty initiation of proteoglycan synthesis causes cardiac and joint defects. Am J Hum Genet. 2011;89:15–27.
von Oettingen JE, Tan WH, Dauber A. Skeletal dysplasia, global developmental delay, and multiple congenital anomalies in a 5-year-old boy-report of the second family with B3GAT3 mutation and expansion of the phenotype. Am J Med Genet A. 2014;164A:1580–6.
Jones KL, Schwarze U, Adam MP, Byers PH, Mefford HC. A homozygous B3GAT3 mutation causes a severe syndrome with multiple fractures, expanding the phenotype of linkeropathy syndromes. Am J Med Genet A. 2015;167A:2691–6.
Budde BS, Mizumoto S, Kogawa R, Becker C, Altmüller J, Thiele H, et al. Skeletal dysplasia in a consanguineous clan from the island of Nias/Indonesia is caused by a novel mutation in B3GAT3. Hum Genet. 2015;134:691–704.
Job F, Mizumoto S, Smith L, Couser N, Brazil A, Saal H, et al. Functional validation of novel compound heterozygous variants in B3GAT3 resulting in severe osteopenia and fractures: expanding the disease phenotype. BMC Med Genet. 2016;17:86.
Yauy K, Tran Mau-Them F, Willems M, Coubes C, Blanchet P, Herlin C, et al. B3GAT3-related disorder with craniosynostosis and bone fragility due to a unique mutation. Genet Med. 2018;20:269–74.
Lin X, Wei G, Shi Z, Dryer L, Esko JD, Wells DE, et al. Disruption of gastrulation and heparan sulfate biosynthesis in EXT1-deficient mice. Dev Biol. 2000;224:299–311.
Takahashi I, Noguchi N, Nata K, Yamada S, Kaneiwa T, Mizumoto S, et al. Important role of heparan sulfate in postnatal islet growth and insulin secretion. Biochem Biophys Res Commun. 2009;383:113–8.
Shimbo M, Suzuki R, Fuseya S, Sato T, Kiyohara K, Hagiwara K, et al. Postnatal lethality and chondrodysplasia in mice lacking both chondroitin sulfate N-acetylgalactosaminyltransferase-1 and -2. PLoS One. 2017;12:e0190333.
Hwang HY, Olson SK, Esko JD, Horvitz HR. Caenorhabditis elegans early embryogenesis and vulval morphogenesis require chondroitin biosynthesis. Nature. 2003;423:439–43.
Mizuguchi S, Uyama T, Kitagawa H, Nomura KH, Dejima K, Gengyo-Ando K, et al. Chondroitin proteoglycans are involved in cell division of Caenorhabditis elegans. Nature. 2003;423:443–8.
Vodopiutz J, Mizumoto S, Lausch E, Rossi A, Unger S, Janocha N, et al. Chondroitin sulfate N-acetylgalactosaminyltransferase-1 (CSGalNAcT-1) deficiency results in a mild skeletal dysplasia and joint laxity. Hum Mutat. 2017;38:34–8.
Mizumoto S, Janecke AR, Sadeghpour A, Povysil G, McDonald MT, Unger S, et al. CSGALNACT1-congenital disorder of glycosylation: A mild skeletal dysplasia with advanced bone age. Hum Mutat. 2020;41:655–67.
Watanabe Y, Takeuchi K, Higa Onaga S, Sato M, Tsujita M, Abe M, et al. Chondroitin sulfate N-acetylgalactosaminyltransferase-1 is required for normal cartilage development. Biochem J. 2010;432:47–55.
Sato T, Kudo T, Ikehara Y, Ogawa H, Hirano T, Kiyohara K, et al. Chondroitin sulfate N-acetylgalactosaminyltransferase 1 is necessary for normal endochondral ossification and aggrecan metabolism. J Biol Chem. 2011;286:5803–12.
Ida-Yonemochi H, Morita W, Sugiura N, Kawakami R, Morioka Y, Takeuchi Y, et al. Craniofacial abnormality with skeletal dysplasia in mice lacking chondroitin sulfate N-acetylgalactosaminyltransferase-1. Sci Rep. 2018;8:17134.
Fawcett JW, Fyhn M, Jendelova P, Kwok JCF, Ruzicka J, Sorg BA. The extracellular matrix and perineuronal nets in memory. Mol Psychiatry. 2022;27:3192–203.
Yoshioka N, Miyata S, Tamada A, Watanabe Y, Kawasaki A, Kitagawa H, et al. Abnormalities in perineuronal nets and behavior in mice lacking CSGalNAcT1, a key enzyme in chondroitin sulfate synthesis. Mol Brain. 2017;10:47.
Li Y, Laue K, Temtamy S, Aglan M, Kotan LD, Yigit G, et al. Temtamy preaxial brachydactyly syndrome is caused by loss-of-function mutations in chondroitin synthase 1, a potential target of BMP signaling. Am J Hum Genet. 2010;87:757–67.
Tian J, Ling L, Shboul M, Lee H, O’Connor B, Merriman B, et al. Loss of CHSY1, a secreted FRINGE enzyme, causes syndromic brachydactyly in humans via increased NOTCH signaling. Am J Hum Genet. 2010;87:768–78.
Li L, Krantz ID, Deng Y, Genin A, Banta AB, Collins CC, et al. Alagille syndrome is caused by mutations in human Jagged1, which encodes a ligand for Notch1. Nat Genet. 1997;16:243–51.
Le Pennec J, Makshakova O, Nevola P, Fouladkar F, Gout E, Machillot P, et al. Glycosaminoglycans exhibit distinct interactions and signaling with BMP2 according to their nature and localization. Carbohydr Polym. 2024;341:122294.
Klüppel M, Wight TN, Chan C, Hinek A, Wrana JL. Maintenance of chondroitin sulfation balance by chondroitin-4-sulfotransferase 1 is required for chondrocyte development and growth factor signaling during cartilage morphogenesis. Development. 2005;132:3989–4003.
Dathe K, Kjaer KW, Brehm A, Meinecke P, Nürnberg P, Neto JC, et al. Duplications involving a conserved regulatory element downstream of BMP2 are associated with brachydactyly type A2. Am J Hum Genet. 2009;84:483–92.
Tan TY, Gonzaga-Jauregui C, Bhoj EJ, Strauss KA, Brigatti K, Puffenberger E, et al. Monoallelic BMP2 Variants Predicted to Result in Haploinsufficiency Cause Craniofacial, Skeletal, and Cardiac Features Overlapping Those of 20p12 Deletions. Am J Hum Genet. 2017;101:985–94.
Wilson DG, Phamluong K, Lin WY, Barck K, Carano RA, Diehl L, et al. Chondroitin sulfate synthase 1 (Chsy1) is required for bone development and digit patterning. Dev Biol. 2012;363:413–25.
Cortes M, Baria AT, Schwartz NB. Sulfation of chondroitin sulfate proteoglycans is necessary for proper Indian hedgehog signaling in the developing growth plate. Development. 2009;136:1697–706.
Domowicz MS, Cortes M, Henry JG, Schwartz NB. Aggrecan modulation of growth plate morphogenesis. Dev Biol. 2009;329:242–57.
Kan AE, Kozlowski K. New distinct lethal osteosclerotic bone dysplasia (Raine syndrome). Am J Med Genet. 1992;43:860–4.
Simpson MA, Hsu R, Keir LS, Hao J, Sivapalan G, Ernst LM, et al. Mutations in FAM20C are associated with lethal osteosclerotic bone dysplasia (Raine syndrome), highlighting a crucial molecule in bone development. Am J Hum Genet. 2007;81:906–12.
Simpson MA, Scheuerle A, Hurst J, Patton MA, Stewart H, Crosby AH. Mutations in FAM20C also identified in non-lethal osteosclerotic bone dysplasia. Clin Genet. 2009;75:271–6.
Nalbant D, Youn H, Nalbant SI, Sharma S, Cobos E, Beale EG, et al. FAM20: an evolutionarily conserved family of secreted proteins expressed in hematopoietic cells. BMC Genomics. 2005;6:11.
Hao J, Narayanan K, Muni T, Ramachandran A, George A. Dentin matrix protein 4, a novel secretory calcium-binding protein that modulates odontoblast differentiation. J Biol Chem. 2007;282:15357–65.
Tagliabracci VS, Engel JL, Wen J, Wiley SE, Worby CA, Kinch LN, et al. Secreted kinase phosphorylates extracellular proteins that regulate biomineralization. Science. 2012;336:1150–3.
Ishikawa HO, Xu A, Ogura E, Manning G, Irvine KD. The Raine syndrome protein FAM20C is a Golgi kinase that phosphorylates bio-mineralization proteins. PLoS One. 2012;7:e42988.
Tagliabracci VS, Engel JL, Wiley SE, Xiao J, Gonzalez DJ, Nidumanda Appaiah H, et al. Dynamic regulation of FGF23 by Fam20C phosphorylation, GalNAc-T3 glycosylation, and furin proteolysis. Proc Natl Acad Sci USA. 2014;111:5520–5.
Tagliabracci VS, Wiley SE, Guo X, Kinch LN, Durrant E, Wen J, et al. A single kinase generates the majority of the secreted phosphoproteome. Cell. 2015;161:1619–32.
Tagliabracci VS, Pinna LA, Dixon JE. Secreted protein kinases. Trends Biochem Sci. 2013;38:121–30.
Koike T, Mikami T, Shida M, Habuchi O, Kitagawa H. Chondroitin sulfate-E mediates estrogen-induced osteoanabolism. Sci Rep. 2015;5:8994.
Yin X, Cheng H, Lin Y, Fan X, Cui Y, Zhou F, et al. Five regulatory genes detected by matching signatures of eQTL and GWAS in psoriasis. J Dermatol Sci. 2014;76:139–42.
Kuroda Y, Murakami H, Enomoto Y, Tsurusaki Y, Takahashi K, Mitsuzuka K, et al. A novel gene (FAM20B encoding glycosaminoglycan xylosylkinase) for neonatal short limb dysplasia resembling Desbuquois dysplasia. Clin Genet. 2019;95:713–7.
Kitazawa K, Nadanaka S, Kadomatsu K, Kitagawa H. Chondroitin 6-sulfate represses keratinocyte proliferation in mouse skin, which is associated with psoriasis. Commun Biol. 2021;4:114.
Malfait F, Francomano C, Byers P, Belmont J, Berglund B, Black J, et al. The 2017 international classification of the Ehlers-Danlos syndromes. Am J Med Genet C Semin Med Genet. 2017;175:8–26.
Malfait F, Castori M, Francomano CA, Giunta C, Kosho T, Byers PH. The Ehlers-Danlos syndromes. Nat Rev Dis Prim. 2020;6:64.
Müller T, Mizumoto S, Suresh I, Komatsu Y, Vodopiutz J, Dundar M, et al. Loss of dermatan sulfate epimerase (DSE) function results in musculocontractural Ehlers-Danlos syndrome. Hum Mol Genet. 2013;22:3761–72.
Dündar M, Müller T, Zhang Q, Pan J, Steinmann B, Vodopiutz J, et al. Loss of dermatan-4-sulfotransferase 1 function results in adducted thumb-clubfoot syndrome. Am J Hum Genet. 2009;85:873–82.
Miyake N, Kosho T, Mizumoto S, Furuichi T, Hatamochi A, Nagashima Y, et al. Loss-of-function mutations of CHST14 in a new type of Ehlers-Danlos syndrome. Hum Mutat. 2010;31:966–74.
Malfait F, Syx D, Vlummens P, Symoens S, Nampoothiri S, Hermanns-Lê T, et al. Musculocontractural Ehlers-Danlos Syndrome (former EDS type VIB) and adducted thumb clubfoot syndrome (ATCS) represent a single clinical entity caused by mutations in the dermatan-4-sulfotransferase 1 encoding CHST14 gene. Hum Mutat. 2010;31:1233–9.
Maccarana M, Olander B, Malmström J, Tiedemann K, Aebersold R, Lindahl U, et al. Biosynthesis of dermatan sulfate: chondroitin-glucuronate C5-epimerase is identical to SART2. J Biol Chem. 2006;281:11560–8.
Evers MR, Xia G, Kang HG, Schachner M, Baenziger JU. Molecular cloning and characterization of a dermatan-specific N-acetylgalactosamine 4-O-sulfotransferase. J Biol Chem. 2001;276:36344–53.
Mikami T, Mizumoto S, Kago N, Kitagawa H, Sugahara K. Specificities of three distinct human chondroitin/dermatan N-acetylgalactosamine 4-O-sulfotransferases demonstrated using partially desulfated dermatan sulfate as an acceptor: implication of differential roles in dermatan sulfate biosynthesis. J Biol Chem. 2003;278:36115–27.
Syx D, Van Damme T, Symoens S, Maiburg MC, van de Laar I, Morton J, et al. Genetic heterogeneity and clinical variability in musculocontractural Ehlers-Danlos syndrome caused by impaired dermatan sulfate biosynthesis. Hum Mutat. 2015;36:535–47.
Lautrup CK, Teik KW, Unzaki A, Mizumoto S, Syx D, Sin HH, et al. Delineation of musculocontractural Ehlers-Danlos Syndrome caused by dermatan sulfate epimerase deficiency. Mol Genet Genom Med. 2020;8:e1197.
Maccarana M, Kalamajski S, Kongsgaard M, Magnusson SP, Oldberg A, Malmström A. Dermatan sulfate epimerase 1-deficient mice have reduced content and changed distribution of iduronic acids in dermatan sulfate and an altered collagen structure in skin. Mol Cell Biol. 2009;29:5517–28.
Kosho T, Miyake N, Hatamochi A, Takahashi J, Kato H, Miyahara T, et al. A new Ehlers-Danlos syndrome with craniofacial characteristics, multiple congenital contractures, progressive joint and skin laxity, and multisystem fragility-related manifestations. Am J Med Genet A. 2010;152A:1333–46.
Shimizu K, Okamoto N, Miyake N, Taira K, Sato Y, Matsuda K, et al. Delineation of dermatan 4-O-sulfotransferase 1 deficient Ehlers-Danlos syndrome: observation of two additional patients and comprehensive review of 20 reported patients. Am J Med Genet A. 2011;155A:1949–58.
Janecke AR, Li B, Boehm M, Krabichler B, Rohrbach M, Müller T, et al. The phenotype of the musculocontractural type of Ehlers-Danlos syndrome due to CHST14 mutations. Am J Med Genet A. 2016;170A:103–15.
Uehara M, Kosho T, Yamamoto N, Takahashi HE, Shimakura T, Nakayama J, et al. Spinal manifestations in 12 patients with musculocontractural Ehlers-Danlos syndrome caused by CHST14/D4ST1 deficiency (mcEDS-CHST14). Am J Med Genet A. 2018;176:2331–41.
Minatogawa M, Unzaki A, Morisaki H, Syx D, Sonoda T, Janecke AR, et al. Clinical and molecular features of 66 patients with musculocontractural Ehlers-Danlos syndrome caused by pathogenic variants in CHST14 (mcEDS-CHST14). J Med Genet. 2022;59:865–77.
Hirose T, Takahashi N, Tangkawattana P, Minaguchi J, Mizumoto S, Yamada S, et al. Structural alteration of glycosaminoglycan side chains and spatial disorganization of collagen networks in the skin of patients with mcEDS-CHST14. Biochim Biophys Acta Gen Subj. 2019;1863:623–31.
Akyüz N, Rost S, Mehanna A, Bian S, Loers G, Oezen I, et al. Dermatan 4-O-sulfotransferase1 ablation accelerates peripheral nerve regeneration. Exp Neurol. 2013;247:517–30.
Yoshizawa T, Mizumoto S, Takahashi Y, Shimada S, Sugahara K, Nakayama J, et al. Vascular abnormalities in the placenta of Chst14-/- fetuses: implications in the pathophysiology of perinatal lethality of the murine model and vascular lesions in human CHST14/D4ST1 deficiency. Glycobiology. 2018;28:80–89.
Nitahara-Kasahara Y, Mizumoto S, Inoue YU, Saka S, Posadas-Herrera G, Nakamura-Takahashi A, et al. A new mouse model of Ehlers-Danlos syndrome generated using CRISPR/Cas9-mediated genomic editing. Dis Model Mech. 2021;14:dmm048963.
Nitahara-Kasahara Y, Posadas-Herrera G, Mizumoto S, Nakamura-Takahashi A, Inoue YU, Inoue T, et al. Myopathy Associated With Dermatan Sulfate-Deficient Decorin and Myostatin in Musculocontractural Ehlers-Danlos Syndrome: A Mouse Model Investigation. Front Cell Dev Biol. 2021;9:695021.
Hirose T, Mizumoto S, Hashimoto A, Takahashi Y, Yoshizawa T, Nitahara-Kasahara Y, et al. Systematic investigation of the skin in Chst14-/- mice: A model for skin fragility in musculocontractural Ehlers-Danlos syndrome caused by CHST14 variants (mcEDS-CHST14). Glycobiology. 2021;31:137–50.
Kusche-Gullberg M, Kjellén L. Sulfotransferases in glycosaminoglycan biosynthesis. Curr Opin Struct Biol. 2003;13:605–11.
Thiele H, Sakano M, Kitagawa H, Sugahara K, Rajab A, Höhne W, et al. Loss of chondroitin 6-O-sulfotransferase-1 function results in severe human chondrodysplasia with progressive spinal involvement. Proc Natl Acad Sci USA. 2004;101:10155–60.
van Roij MH, Mizumoto S, Yamada S, Morgan T, Tan-Sindhunata MB, Meijers-Heijboer H, et al. Spondyloepiphyseal dysplasia, Omani type: further definition of the phenotype. Am J Med Genet A. 2008;146A:2376–84.
Tuysuz B, Mizumoto S, Sugahara K, Celebi A, Mundlos S, Turkmen S. Omani-type spondyloepiphyseal dysplasia with cardiac involvement caused by a missense mutation in CHST3. Clin Genet. 2009;75:375–83.
Hermanns P, Unger S, Rossi A, Perez-Aytes A, Cortina H, Bonafé L, et al. Congenital joint dislocations caused by carbohydrate sulfotransferase 3 deficiency in recessive Larsen syndrome and humero-spinal dysostosis. Am J Hum Genet. 2008;82:1368–74.
Unger S, Lausch E, Rossi A, et al. Phenotypic features of carbohydrate sulfotransferase 3 (CHST3) deficiency in 24 patients: congenital dislocations and vertebral changes as principal diagnostic features. Am J Med Genet A. 2010;152A:2543–9.
Uchimura K, Kadomatsu K, Nishimura H, Muramatsu H, Nakamura E, Kurosawa N, et al. Functional analysis of the chondroitin 6-sulfotransferase gene in relation to lymphocyte subpopulations, brain development, and oversulfated chondroitin sulfates. J Biol Chem. 2002;277:1443–50.
Kitagawa H, Tsutsumi K, Tone Y, Sugahara K. Developmental regulation of the sulfation profile of chondroitin sulfate chains in the chicken embryo brain. J Biol Chem. 1997;272:31377–81.
Properzi F, Carulli D, Asher RA, Muir E, Camargo LM, van Kuppevelt TH, et al. Chondroitin 6-sulphate synthesis is up-regulated in injured CNS, induced by injury-related cytokines and enhanced in axon-growth inhibitory glia. Eur J Neurosci. 2005;21:378–90.
Mitsunaga C, Mikami T, Mizumoto S, Fukuda J, Sugahara K. Chondroitin sulfate/dermatan sulfate hybrid chains in the development of cerebellum. Spatiotemporal regulation of the expression of critical disulfated disaccharides by specific sulfotransferases. J Biol Chem. 2006;281:18942–52.
Miyata S, Komatsu Y, Yoshimura Y, Taya C, Kitagawa H. Persistent cortical plasticity by upregulation of chondroitin 6-sulfation. Nat Neurosci. 2012;15:414–22.
Chopra SS, Leshchiner I, Duzkale H, McLaughlin H, Giovanni M, Zhang C, et al. Inherited CHST11/MIR3922 deletion is associated with a novel recessive syndrome presenting with skeletal malformation and malignant lymphoproliferative disease. Mol Genet Genomic Med. 2015;3:413–23.
Shabbir RMK, Nalbant G, Ahmad N, Malik S, Tolun A. Homozygous CHST11 mutation in chondrodysplasia, brachydactyly, overriding digits, clino-symphalangism and synpolydactyly. J Med Genet. 2018;55:489–96.
Ohtake-Niimi S, Kondo S, Ito T, Kakehi S, Ohta T, Habuchi H, et al. Mice deficient in N-acetylgalactosamine 4-sulfate 6-o-sulfotransferase are unable to synthesize chondroitin/dermatan sulfate containing N-acetylgalactosamine 4,6-bissulfate residues and exhibit decreased protease activity in bone marrow-derived mast cells. J Biol Chem. 2010;285:20793–805.
Salpietro V, Ruggieri M, Mankad K, Di Rosa G, Granata F, Loddo I, et al. A de novo 0.63 Mb 6q25.1 deletion associated with growth failure, congenital heart defect, underdeveloped cerebellar vermis, abnormal cutaneous elasticity and joint laxity. Am J Med Genet A. 2015;167A:2042–51.
Goossens D, Van Gestel S, Claes S, De Rijk P, Souery D, Massat I, et al. A novel CpG-associated brain-expressed candidate gene for chromosome 18q-linked bipolar disorder. Mol Psychiatry. 2003;8:83–9.
Zayed H, Chao R, Moshrefi A, Lopezjimenez N, Delaney A, Chen J, et al. A maternally inherited chromosome 18q22.1 deletion in a male with late-presenting diaphragmatic hernia and microphthalmia-evaluation of DSEL as a candidate gene for the diaphragmatic defect. Am J Med Genet A. 2010;152A:916–23.
Funding
This work was supported in part by a Grant-in-Aid for Scientific Research (C) 23K06142 (to SM), 24K09372 (to TM), and 24K09805 (to SY), and Scientific Research (B) 24K02183 (to HK) from the Japan Society for the Promotion of Science, Japan.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mikami, T., Mizumoto, S., Kitagawa, H. et al. Congenital disorders caused by aberrations in the biosynthesis of chondroitin/dermatan sulfate. J Hum Genet (2025). https://doi.org/10.1038/s10038-025-01396-0
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s10038-025-01396-0