Abstract
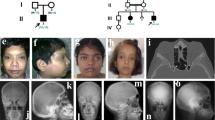
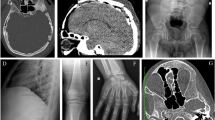
Transmembrane protein 53 (TMEM53) is an outer nuclear membrane protein that plays a crucial role in maintaining skeletal homeostasis. Pathogenic variants in TMEM53 have been identified as the genetic cause of craniotubular dysplasia, Ikegawa type (CTDI), a rare form of sclerosing bone dysplasia characterized by skull hyperostosis, cranial deformities, and increased bone density. To date, the causal association of bi-allelic pathogenic variants of TMEM53 in CTDI has been identified in 14 patients from eight unrelated families. Mechanistically, TMEM53 negatively regulates BMP–SMAD signaling by restricting the nuclear import of phosphorylated SMAD1/5/9, thereby modulating osteoblast differentiation and bone formation. This review summarizes the current understanding of TMEM53 function and the consequences of its deficiency. We aim to clarify genotype-phenotype correlations, outline therapeutic prospects for CTDI, and explore the distinct mechanisms underlying cranial and tubular bone formation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Korfali N, Srsen V, Waterfall M, Batrakou DG, Pekovic V, Hutchison CJ, et al. A flow cytometry-based screen of nuclear envelope transmembrane proteins identifies NET4/tmem53 as involved in stress-dependent cell cycle withdrawal. PLoS One. 2011;6:e18762.
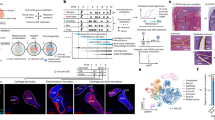
Guo L, Iida A, Bhavani GSL, Gowrishankar K, Wang Z, Xue J yi, et al. Deficiency of TMEM53 causes a previously unknown sclerosing bone disorder by dysregulation of BMP-SMAD signaling. Nat Commun. 2021;12:2046.
Herrera-Quiterio GA, Encarnación-Guevara S. The transmembrane proteins (TMEM) and their role in cell proliferation, migration, invasion, and epithelial-mesenchymal transition in cancer. Front Oncol. 2023;13:1244740.
Xu H, Yang S, Liu P, Zhang Y, Zhang T, Lan J, et al. The roles and functions of TMEM protein family members in cancers, cardiovascular and kidney diseases (Review). Biomed Rep. 2025;22:63.
Graves AR, Curran PK, Smith CL, Mindell JA. The Cl-/H+ antiporter ClC-7 is the primary chloride permeation pathway in lysosomes. Nature. 2008;453:788–92.
Xue JY, Grigelioniene G, Wang Z, Nishimura G, Iida A, Matsumoto N, et al. SLC4A2 deficiency causes a new type of osteopetrosis. J Bone Miner Res. 2020;37:226–35.
Heinemann T, Bulwin GC, Randall J, Schnieders B, Sandhoff K, Volk HD, et al. Genomic organization of the gene coding for TIRC7, a novel membrane protein essential for T cell activation. Genomics. 1999;57:398–406.
Lv, Xu F, Wang XJ, Liu JY, Asan Y, Wang JW, et al. Two novel mutations in TMEM38B result in rare autosomal recessive osteogenesis imperfecta. J Hum Genet. 2016;61:539–45.
Dougall WC, Glaccum M, Charrier K, Rohrbach K, Brasel K, De Smedt T, et al. RANK is essential for osteoclast and lymph node development. Genes Dev. 1999;13:2412–24.
Mao J, Wang J, Liu B, Pan W, Farr GH, Flynn C, et al. Low-density lipoprotein receptor-related protein-5 binds to axin and regulates the Canonical Wnt signaling pathway. Mol Cell. 2001;7:801–9.
Luan X, Lu Q, Jiang Y, Zhang S, Wang Q, Yuan H, et al. Crystal structure of human RANKL complexed with its decoy receptor osteoprotegerin. J Immunol. 2012;189:245–52.
Kawao N, Matsumura D, Yamada A, Okumoto K, Ohira T, Mizukami Y, et al. Tmem119 is involved in bone anabolic effects of PTH through enhanced osteoblastic bone formation in mice. Bone. 2024;181:117040.
Liu J, Bao X, Huang J, Chen R, Tan Y, Zhang Z, et al. TMEM135 maintains the equilibrium of osteogenesis and adipogenesis by regulating mitochondrial dynamics. Metabolism. 2024;152:155767.
Decker CE, Yang Z, Rimer R, Park-Min KH, Macaubas C, Mellins ED, et al. Tmem178 acts in a novel negative feedback loop targeting NFATc1 to regulate bone mass. Proc. Natl Acad. Sci. 2015;112:15654–9.
Janin A, Bauer D, Ratti F, Millat G, Méjat A. Nuclear envelopathies: a complex LINC between nuclear envelope and pathology. Orphanet J Rare Dis. 2017;12:147.
Kim H, Kim T, Jeong BC, Cho IT, Han D, Takegahara N, et al. Tmem64 modulates calcium signaling during RANKL-mediated osteoclast differentiation. Cell Metab. 2013;17:249–60.
McEwan DG, Popovic D, Gubas A, Terawaki S, Suzuki H, Stadel D, et al. PLEKHM1 regulates autophagosome-lysosome fusion through HOPS complex and LC3/GABARAP Proteins. Mol Cell. 2015;57:39–54.
Vacher J. OSTM1 pleiotropic roles from osteopetrosis to neurodegeneration. Bone. 2022;163:116505.
Aranda V, Martı́nez I, Melero S, Lecanda J, Banales JM, Prieto J, et al. Shared apical sorting of anion exchanger isoforms AE2a, AE2b1, and AE2b2 in primary hepatocytes. Biochem Biophys Res Commun. 2004;319:1040–6.
Sirinian C, Papanastasiou AD, Zarkadis IK, Kalofonos HP. Alternative splicing generates a truncated isoform of human TNFRSF11A (RANK) with an altered capacity to activate NF-κB. Gene. 2013;525:124–9.
Shaheen R, Alazami AM, Alshammari MJ, Faqeih E, Alhashmi N, Mousa N, et al. Study of autosomal recessive osteogenesis imperfecta in Arabia reveals a novel locus defined by TMEM38B mutation. J Med Genet. 2012;49:630–5.
Debeer P, Pykels E, Lammens J, Devriendt K, Fryns J-P. Melorheostosis in a family with autosomal dominant osteopoikilosis: Report of a third family. Am J Med Genet A. 2003;119A:188–93.
Schirmer EC, Florens L, Guan T, Yates JR, Gerace L. Nuclear membrane proteins with potential disease links found by subtractive proteomics. Science. 2003;301:1380–2.
Ren K, Pirmarzdashti N, Pakdel F, Zhu J, Liu W, Wang L, et al. A novel missense pathogenic variants of TMEM53 in an Iranian family with craniotubular dysplasia, Ikegawa type. J Hum Genet. 2025;7:195−8.
Whyte MP, Weinstein RS, Phillips PH, McAlister WH, Ramakrishnaiah RH, Schaefer GB, et al. Transmembrane protein 53 craniotubular dysplasia (OMIM # 619727): the skeletal disease and consequent blindness of this new disorder. Bone. 2024;188:117218.
van Ommeren B, Hoekstra M, van Gassen K, van Jaarsveld R, van Haaften G, Mathijssen I, et al. Craniotubular Dysplasia Ikegawa Type: Further Delineation of the Phenotype. Am J Med Genet A. 2024;197:e63870.
Peng Y, Wan Z, Li K, Liu Z, Chen J, Hu A, et al. Novel TMEM53 missense variant generated a new ubiquitination site and cause Craniotubular dysplasia, Ikegawa type. Hum Mol Genet. 2025;34:1592−8.
Acknowledgements
We thank the patients and their families for their help to the study.
Funding
This study was supported by National Natural Science Foundation of China (Grant No. 82471900, L.G.) (Grant No. 82201865, Y.L.) (Grant No. 32400088, D.X.), Supporting Program of Innovation Capability Shaanxi Province (2024CX-GXPT-40, L.G.), Young Talent Support Plan from Xi’an Jiaotong University (YX6J033, L.G.), and Provincial Talent Research Support Fund (71240000000131, L.G.), Shaanxi Provincial Project for Innovation Capacity Improvement of Health Research (2024PT-03, R.Q.), and Provincial Key Industry Innovation Chain (2024SF-ZDCYL-04-05, R.Q.), Department of Genetics, NHC Key Laboratory of Healthy Birth and Birth Defect Prevention in Western China First People's Hospital of Yunnan Province, Kunming, China (No.2025XBYSKF012) Grant for NHC Key Laboratory of Healthy Birth and Birth Defect Prevention in Western China First People’s Hospital of Yunnan Province (L.W.).
Author information
Authors and Affiliations
Contributions
Long Guo and Shiro Ikegawa conceived and designed the review. Kaitao Ren drafted the initial manuscript; all authors contributed to subsequent revisions and critically reviewed the content. All authors approved the final version for submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ren, K., Fu, Y., Zhu, J. et al. TMEM53 as an outer nuclear membrane regulator of cranial and tubular bone formation in craniotubular dysplasia. J Hum Genet (2025). https://doi.org/10.1038/s10038-025-01443-w
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s10038-025-01443-w