Abstract
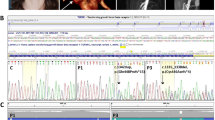
Recent studies have revealed de novo or germline-derived GNAS-Gsα variants with constitutive ligand-independent gain-of-function (GOF) effects on specific G-protein-coupled receptor signalings in patients with nephrogenic syndrome of inappropriate antidiuresis (NSIAD), osteolytic bone disorder with metaphyseal dysplasia, and peripheral precocious puberty. We encountered a Japanese girl with NSIAD and osteolytic bone disorder with metaphyseal dysplasia. Whole genome sequencing identified a de novo ″likely pathogenic″ heterozygous GNAS-Gsα missense variant (NM_000516.7:c.163 A > G:p.(Thr55Ala)) which occurred on the paternally inherited allele. Luciferase assays for p.Thr55Ala showed ligand-independent GOF effects on AVPR2 and PTH1R signalings, and a ligand-dependent loss-of-function (LOF) effect on PTH1R signaling. Protein structural analysis for p.Thr55Ala indicated disruption of the hydrogen bond between p.Thr55 side chain and the α-phosphate group of the bound nucleotide in both GDP-bound inactive form and GTP-bound active form and resultantly reduced affinity of the variant-positive Gsα protein for both GDP and GTP, consistent with the ligand-independent GOF and ligand-dependent LOF effects. The results, in conjunction with the previous findings, indicate that GNAS-Gsα variants with constitutive GOF effects cause clinically distinctive congenital rare disorders including NSIAD and characteristic bone disorder.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Weinstein LS, Yu S, Warner DR, Liu J. Endocrine manifestations of stimulatory G protein α-subunit mutations and the role of genomic imprinting. Endocr Rev. 2001;22:675–705.
Turan S, Bastepe MTheGNAS. complex locus and human diseases associated with loss-of-function mutations or epimutations within this imprinted gene. Horm Res Paediatr. 2013;80:229–41.
Spiegel AM, Weinstein LS. Inherited diseases involving G proteins and G protein-coupled receptors. Annu Rev Med. 2004;55:27–39.
Yu S, Yu D, Lee E, Eckhaus M, Lee R, Corria Z, et al. Variable and tissue-specific hormone resistance in heterotrimeric Gs protein alpha-subunit (Gsα) knockout mice is due to tissue-specific imprinting of the Gsα gene. Proc Natl Acad Sci USA. 1998;95:8715–20.
Hayward BE, Barlier A, Korbonits M, Grossman AB, Jacquet P, Enjalbert A, et al. Imprinting of the Gsα gene GNAS1 in the pathogenesis of acromegaly. J Clin Invest. 2001;107:R31-6.
Mantovani G, Ballare E, Giammona E, Beck-Peccoz P, Spada A. The Gsα gene: predominant maternal origin of transcription in human thyroid gland and gonads. J Clin Endocrinol Metab. 2002;87:4736–40.
Elli FM, de Sanctis L, Ceoloni B, Barbieri AM, Bordogna P, Beck-Peccoz P, et al. Pseudohypoparathyroidism type Ia and pseudo-pseudohypoparathyroidism: the growing spectrum of GNAS inactivating mutations. Hum Mutat. 2013;34:411–6.
Weinstein LS, Shenker A, Gejman PV, Merino MJ, Friedman E, Spiegel AM. Activating mutations of the stimulatory G protein in the McCune-Albright syndrome. N Engl J Med. 1991;325:1688–95.
Miyado M, Fukami M, Takada S, Terao M, Nakabayashi K, Hata K, et al. Germline-derived gain-of-function variants of Gsα-coding GNAS gene identified in nephrogenic syndrome of inappropriate antidiuresis. J Am Soc Nephrol. 2019;30:877–89.
Biebermann H, Kleinau G, Schnabel D, Bockenhauer D, Wilson LC, Tully I, et al. A new multisystem disorder caused by the Gαs mutation p.F376V. J Clin Endocrinol Metab. 2019;104:1079–89.
Cavarzere P, Gastaldi A, Elli FM, Gaudino R, Peverelli E, Brugnara M, et al. A complex phenotype in a girl with a novel heterozygous missense variant (p.Ile56Phe) of the GNAS gene. Orphanet J Rare Dis. 2022;17:83.
Carcavilla A, Pereda A, Miyado M, Fukami M, Kato F, Sengoku T, et al. Germline-derived GNAS-Gsα variants associated with both gain-of-function and loss-of-function phenotypes. Eur J Endocrinol. 2025;192:364–72.
Iiri T, Herzmark P, Nakamoto JM, Van Dop C, Bourne HR. Rapid GDP release from Gsα in patients with gain and loss of endocrine function. Nature. 1997;371:164–8.
Nakamoto JM, Zimmerman D, Jones EA, Loke KY, Siddiq K, Donlan MA, et al. Concurrent hormone resistance (pseudohypoparathyroidism type Ia) and hormone independence (testotoxicosis) caused by a unique mutation in the Gαs gene. Biochem Mol Med. 1996;58:18–24.
Wentworth K, Hsing A, Urrutia A, Zhu Y, Horvai AE, Bastepe M, et al. A novel T55A variant of Gsα associated with impaired cAMP production, bone fragility, and osteolysis. Case Rep Endocrinol. 2016;2016:2691385.
Resch B, Resch E, Maurer-Fellbaum U, Pichler-Stachl E, Riccabona M, Hofer N, et al. The whole spectrum of cystic periventricular leukomalacia of the preterm infant: results from a large consecutive case series. Childs Nerv Syst. 2015;31:1527–32.
Feldman BJ, Rosenthal SM, Vargas GA, Fenwick RG, Huang EA, Matsuda-Abedini M, et al. Nephrogenic syndrome of inappropriate antidiuresis. N Engl J Med. 2005;352:1884–90.
Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–75.
Liu X, Xu X, Hilger D, Aschauer P, Tiemann JKS, Du Y, et al. Structural insights into the process of GPCR-G protein complex formation. Cell. 2019;177:1243–51.e12.
Dai SA, Hu Q, Gao R, Blythe EE, Touhara KK, Peacock H, et al. State-selective modulation of heterotrimeric Gsα signaling with macrocyclic peptides. Cell. 2022;185:3950–65.e1–12.
Pai EF, Kabsch W, Krengel U, Holmes KC, John J, Wittinghofer A. Structure of the guanine-nucleotide-binding domain of the Ha-ras oncogene product p21 in the triphosphate conformation. Nature. 1989;341:209–14.
Bockenhauer D, Bichet DG. Pathophysiology, diagnosis and management of nephrogenic diabetes insipidus. Nat Rev Nephrol. 2015;11:576–88.
Unger S, Ferreira CR, Mortier GR, Ali H, Bertola DR, Calder A, et al. Nosology of genetic skeletal disorders: 2023 revision. Am J Med Genet A. 2023;191:1164–209.
Obiezu F, Boyce A, Jüppner H, Jha S. PTH1R-related Jansen metaphyseal chondrodysplasia. In: Adam MP, Feldman J, Mirzaa GM, et al. editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993–2025Bookshelf ID: NBK6 16087.
Bilezikian JP, Khan AA, Silverberg SJ, Fuleihan GE, Marcocci C, Minisola S, et al. Evaluation and management of primary hyperparathyroidism: summary statement and guidelines from the Fifth International Workshop. J Bone Miner Res. 2022;37:2293–314.
Gray MJ, van Kogelenberg M, Beddow R, Morgan T, Wordsworth P, Shears DJ, et al. A new acro-osteolysis syndrome caused by duplications including PTHLH. J Hum Genet. 2014;59:484–7.
Tacke CE, Terheggen-Lagro SWJ, Boot AM, Plomp AS, Polstra AM, van Rijn RR, et al. Chondrodysplasia, enchondromas and a chest deformity causing severe pulmonary morbidity in a boy with a PTHLH duplication: a case report. Bone Rep. 2021;14:101067.
Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405–24.
Flock T, Hauser AS, Lund N, Gloriam DE, Balaji S, Babu MM. Selectivity determinants of GPCR-G-protein binding. Nature. 2017;545:317–22.
Strachan T, Raed A. Human genetic variability and its consequences. In: Human molecular genetics, 4th ed. Garland Science, Taylor and Francis Group. LLC, 2011:405–40.
Tajan M, Paccoud R, Branka S, Edouard T, Yart A. The RASopathy family: consequences of germline activation of the RAS/MAPK pathway. Endocr Rev. 2018;39:676–700.
Weitil N, Seifert R. Distinct interactions of human β1- and β2-adrenoceptors with isoproterenol, epinephrine, norepinephrine, and dopamine. J Pharmacol Exp Ther. 2008;327:760–9.
Resch B, Vollaard E, Maurer U, Haas J, Rosegger H, Müller W. Risk factors and determinants of neurodevelopmental outcome in cystic periventricular leukomalacia. Eur J Pediatr. 2000;159:663–70.
Azam S, Haque ME, Jakaria M, Jo SH, Kim IS, Choi DK. G-protein-coupled receptors in CNS: a potential therapeutic target for intervention in neurodegenerative disorders and associated cognitive deficits. Cells. 2020;9:506.
Chen X, Meng Y, Tang M, Wang Y, Xie Y, Wan S, et al. A paternally inherited non-sense variant c.424G>T (p.G142*) in the first exon of XLalphas in an adult patient with hypophosphatemia and osteopetrosis. Clin Genet. 2020;97:712–22.
Acknowledgements
We would like to thank Dr. Mami Miyado, Dr. Maki Fukami, and Ms. Fumiko Kato for their advice in the luciferase assays.
Funding
This work was supported by the grant from Japan Agency for Medical Research and Development (AMED) (JP25ek0109805, JP25ek0109760, JP24ek0109760, JP25ek0109674, and JP24ek0109587), by the Japanese Society for Pediatric Endocrinology Future Development Grant supported by Novo Nordisk Pharma Ltd, and by a research grant from Astellas Pharma.
Author information
Authors and Affiliations
Contributions
MI performed molecular data curation and functional studies. CN obtained clinical data and blood samples. GN evaluated skeletal findings and supervised this study. NS and JT performed whole genome sequencing. YF performed functional studies and supervised this study. TS and KO carried out protein structural analysis, HS performed molecular data curation and supervised this study. TO conceptualized this study, obtained clinical data, and wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ikeda, M., Numakura, C., Nishimura, G. et al. De novo GNAS-Gsα variant (p.Thr55Ala) with constitutive gain-of-function effects on AVPR2 and PTH1R signalings. J Hum Genet (2026). https://doi.org/10.1038/s10038-025-01448-5
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s10038-025-01448-5