Abstract
Background
Elevated adiposity is often posited by medical and public health researchers to be a risk factor associated with cardiovascular disease, diabetes, and other diseases. These health challenges are now thought to be reflected in epigenetic modifications to DNA molecules, such as DNA methylation, which can alter gene expression.
Methods
Here we report the results of three Epigenome Wide Association Studies (EWAS) in which we assessed the differential methylation of DNA (obtained from peripheral blood) associated with three adiposity phenotypes (BMI, waist circumference, and impedance-measured percent body fat) among American Indian adult participants in the Strong Heart Study.
Results
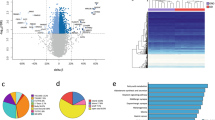
We found differential methylation at 8264 CpG sites associated with at least one of our three response variables. Of the three adiposity proxies we measured, waist circumference had the highest number of associated differentially methylated CpGs, while percent body fat was associated with the lowest. Because both waist circumference and percent body fat relate to physiology, we focused interpretations on these variables. We found a low degree of overlap between these two variables in our gene ontology enrichment and Differentially Methylated Region analyses, supporting that waist circumference and percent body fat measurements represent biologically distinct concepts.
Conclusions
We interpret these general findings to indicate that highly significant regions of the genome (DMR) and synthesis pathways (GO) in waist circumference analyses are more likely to be associated with the presence of visceral/abdominal fat than more general measures of adiposity. Our findings confirmed numerous CpG sites previously found to be differentially methylated in association with adiposity phenotypes, while we also found new differentially methylated CpG sites and regions not previously identified.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
Access to Strong Heart Study raw data is subject to approval by participating tribes following the procedures established by the Strong Heart Study in agreement with the tribes. Detailed information is available in the Strong Heart Study website https://strongheartstudy.org/.
Code availability
Code used to analyze data in this paper is available from the senior author and the corresponding author.
References
Wahl S, Drong A, Lehne B, Loh M, Scott WR, Kunze S, et al. Epigenome-wide association study of body mass index, and the adverse outcomes of adiposity. Nature. 2017;541:81.
Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:766–81.
Dhana K, Braun K, Nano J, Voortman R, Joyce M, Uitterlinden A, et al. An epigenome-wide association study (EWAS) of obesity-related traits. Annals Nutr Metab. 2017;71:287–287.
Shai I, Jiang R, Manson JE, Stampler MJ, Willett WC, Colditz GA, et al. Ethnicity, obesity, and risk of type 2 diabetes in women - A 20-year follow-up study. Diabetes Care. 2006;29:1585–90.
Deurenberg-Yap M, Schmidt G, van Staveren WA, Deurenberg P. The paradox of low body mass index and high body fat percentage among Chinese, Malays and Indians in Singapore. Int J Obes. 2000;24:1011–7.
Rush EC, Goedecke J, Jennings C, Micklesfield L, Dugas L, Lambert EV, et al. BMI, fat and muscle differences in urban women of five ethnicities from two countries. Int J Obes. 2007;31:1232–9.
Muller MJ, Geisler C, Blundell J, Dulloo A, Schutz Y, Krawczak M. The case of GWAS of obesity: does body weight control play by the rules? Int J Obes. 2018;42:1395–405.
Bosy-Westphal A, Braun W, Geisler C, Norman K, Muller MJ. Body composition and cardiometabolic health: the need for novel concepts. Eur J Clin Nutr. 2018;72:638–44.
Demerath EW, Guan WH, Grove ML, Aslibekyan S, Mendelson M, Zhou YH, et al. Epigenome-wide association study (EWAS) of BMI, BMI change and waist circumference in African American adults identifies multiple replicated loci. Human Mol Gen. 2015;24:4464–79.
Al Muftah WA, Al-Shafai M, Zaghlool SB, Visconti A, Tsai PC, Kumar P, et al. Epigenetic associations of type 2 diabetes and BMI in an Arab population. Clin Epigen. 2016;8:13.
Meeks KAC, Henneman P, Venema A, Burr T, Galbete C, Danquah I, et al. An epigenome-wide association study in whole blood of measures of adiposity among Ghanaians: the RODAM study. Clin Epigen. 2017;9:103.
Schlesinger S, Aleksandrova K, Pischon T, Fedirko V, Jenab M, Trepo E, et al. Abdominal obesity, weight gain during adulthood and risk of liver and biliary tract cancer in a European cohort. Int J Cancer. 2013;132:645–57.
Pang YJ, Kartsonaki C, Guo Y, Chen YP, Yang L, Bian Z, et al. Central adiposity in relation to risk of liver cancer in Chinese adults: a prospective study of 0.5 million people. Int J Cancer. 2019;145:1245–53.
Campbell PT, Newton CC, Freedman ND, Koshiol J, Alavanja MC, Freeman LEB, et al. Body mass index, waist circumference, diabetes, and risk of liver cancer for US adults. Cancer Res. 2016;76:6076–83.
Shuster A, Patlas M, Pinthus JH, Mourtzakis M. The clinical importance of visceral adiposity: a critical review of methods for visceral adipose tissue analysis. Br J Radiol. 2012;85:1–10.
Lee ET, Welty TK, Fabsitz R, Cowan LD, Le NA, Oopik AJ, et al. The strong heart study - a study of cardiovascular disease in American Indians: designs and methods. Am J Epidemiol. 1990;132:1141–55.
The Strong Heart Study Manual Chapter 5: Clinical Examination. 2012 (Modified May 2, 2003). https://strongheartstudy.org/portals/1288/Assets/documents/manuals/Phase%20I%20Operations%20Manual.pdf?ver=2017-11-15-134631-313.
Rising R, Swinburn B, Larson K, Ravussin E. Body composition in Pima Indians: validation of bioelectrical resistance. Am J Clin Nutr. 1991;53:594–8.
Stolarczyk LM, Heyward VH, Hicks VL, Baumgartner RN. Predictive accuracy of bioelectrical impedance in estimating body composition of Native American women. Am J Clin Nutr. 1994;59:964–70.
Fortin JP, Triche TJ, Hansen KD. Preprocessing, normalization and integration of the Illumina HumanMethylationEPIC array with minfi. Bioinformatics. 2017;33:558–60.
Triche TJ, Weisenberger DJ, Van Den Berg D, Laird PW, Siegmund KD. Low-level processing of Illumina Infinium DNA Methylation BeadArrays. Nucleic Acids Res. 2013;41:e90.
Houseman EA, Accomando WP, Koestler DC, Christensen BC, Marsit CJ, Nelson HH, et al. DNA methylation arrays as surrogate measures of cell mixture distribution. Bmc Bioinform. 2012;13:86.
McCartney DL, Walker RM, Morris SW, McIntosh AM, Porteous DJ, Evans KL. Identification of polymorphic and off-target probe binding sites on the Illumina Infinium MethylationEPIC BeadChip. Genomics Data. 2016;9:22–24.
Ritchie ME, Phipson B, Wu D, Hu YF, Law CW, Shi W, et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucl Acids Res. 2015;43:e47.
Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B-Methodol. 1995;57:289–300.
Demerath NJ, The American Soldier: Volume I, adjustment during army life. By S. A. Stouffer, E. A. Suchman, L. C. DeVinney, S. A. Star, R. M. Williams, Jr. Volume II, Combat and Its Aftermath. By S. A. Stouffer, A. A. Lumsdaine, M. H. Lumsdaine, R. M. Williams, Jr., M. B. Smith, I. L. Janis, S. A. Star, L. S. Cottrell, Jr. Princeton, New Jersey: Princeton University Press, 1949. Vol. I, 599 pp., Vol. II, 675 pp. $7.50 each; $13.50 together. Social Forces. 1949;28:87–90.
Afshin A, Forouzanfar MH, Reitsma MB, Sur P, Estep K, Lee A, et al. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med. 2017;377:13–27.
Gonzalez MC, Correia M, Heymsfield SB. A requiem for BMI in the clinical setting. Current Opin Clin Nutr Metab Care. 2017;20:314–21.
Gearon E, Tanamas SK, Stevenson C, Loh VHY, Peeters A. A., Changes in waist circumference independent of weight: Implications for population level monitoring of obesity. Preventive Med. 2018;111:378–83.
Prentice AM, Jebb SA. Beyond body mass index. Obes Rev. 2001;2:141–7.
Martí A, Marcos A, Martínez JA. Obesity and immune function relationships. Obes Rev. 2001;2:131–40.
O’Rourke RW, Metcalf MD, White AE, Madala A, Winters BR, Maizlin II, et al. Depot-specific differences in inflammatory mediators and a role for NK cells and IFN-gamma in inflammation in human adipose tissue. Int J Obes. 2009;33:978–90.
Lappalainen T, Greally JM. Associating cellular epigenetic models with human phenotypes. Nat Rev Gen. 2017;18:441–51.
Moore LD, Le T, Fan G. DNA methylation and its basic function. Neuropsychopharmacology. 2013;38:23–38.
Buchta CM, Boi KS, Miller BJ, Milhem MM, Norian LA. Obesity does not exacerbate the protumorigenic systemic environment in sarcoma subjects. ImmunoHorizons. 2017;1:20.
Joffe L, Dwyer S, Bender JLG, Frazier AL, Ladas EJ. Nutritional status and clinical outcomes in pediatric patients with solid tumors: A systematic review of the literature. Sem Oncol. 2019;46:48–56.
Choromanska B, Mysliwiec P, Hady HR, Dadan J, Mysliwiec H, Bonda T, et al. The implication of adipocyte ATP-binding cassette A1 and G1 transporters in metabolic complications of obesity. J Physiol Pharmacol. 2019;70:143–52.
Samaras K, Botelho NK, Chisholm DJ, Lord RV. Subcutaneous and visceral adipose tissue gene expression of serum adipokines that predict type 2 diabetes. Obesity. 2010;18:884–9.
Pateyjohns IR, Brinkworth GD, Buckley JD, Noakes M, Clifton PM. Comparison of three bioelectrical impedance methods with DXA in overweight and obese men. Obesity. 2006;14:2064–70.
Borga M, West J, Bell JD, Harvey NC, Romu T, Heymsfield SB, et al. Advanced body composition assessment: from body mass index to body composition profiling. J Invest Med. 2018;66:887–95.
Hunger JM, Major B. Weight stigma mediates the association between bmi and self-reported health. Health Psych. 2015;34:172–5.
Pause C. Borderline: the ethics of fat stigma in public health. J Law Med Ethics. 2017;45:510–7.
Hill JH, Solt C, Foster MT. Obesity associated disease risk: the role of inherent differences and location of adipose depots. Hormone Molecular Biol Clin Invest. 2018;33.
Black RE, Allen LH, Bhutta ZA, Caulfield LE, de Onis M, Ezzati M, et al. Maternal and child undernutrition 1—maternal and child undernutrition: global and regional exposures and health consequences. Lancet. 2008;371:243–60.
Miller SL, Wolfe RR. The danger of weight loss in the elderly. J Nutr Health Aging. 2008;12:487–91.
Wells JCK, Fewtrell MS. Measuring body composition. Arch Dis Child. 2006;91:612–7.
Klopfenstein BJ, Kim MS, Krisky CM, Szumowski J, Rooney WD, Purnell JQ. Comparison of 3 T MRI and CT for the measurement of visceral and subcutaneous adipose tissue in humans. Br J Radiol. 2012;85:e826–e830.
Barcelona V, Huang YF, Brown K, Liu JX, Zhao W, Yu M, et al. Novel DNA methylation sites associated with cigarette smoking among African Americans. Epigenetics. 2019;14:383–91.
Fasanelli F, Baglietto L, Ponzi E, Guida F, Campanella G, Johansson M, et al. Hypomethylation of smoking-related genes is associated with future lung cancer in four prospective cohorts. Nat Commun. 2015;6:10192.
Leek JT, Johnson WE, Parker HS, Jaffe AE, Storey JD. The sva package for removing batch effects and other unwanted variation in high-throughput experiments. Bioinformatics. 2012;28:882–3.
Acknowledgements
This work was supported by grants for the National Heart, Lung, and Blood Institute (NHLBI) (under contract numbers 75N92019D00027, 75N92019D00028, 75N92019D00029, and 75N92019D00030) and previous grants (R01HL090863, R01HL109315, R01HL109301, R01HL109284, R01HL109282, and R01HL109319 and cooperative agreements: U01HL41642, U01HL41652, U01HL41654, U01HL65520, and U01HL65521) and by the National Institutes of Health Sciences (R01ES021367, R01ES025216, P42ES010349, P30ES009089). Thanks to Dr. Pascale R Leroueil for computational expertise and Ali Thompson for illuminating discussions. All analyses and writing of this manuscript were done on the occupied territory of the Lenape and Wappinger Nations.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Crocker, K.C., Domingo-Relloso, A., Haack, K. et al. DNA methylation and adiposity phenotypes: an epigenome-wide association study among adults in the Strong Heart Study. Int J Obes 44, 2313–2322 (2020). https://doi.org/10.1038/s41366-020-0646-z
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41366-020-0646-z
This article is cited by
-
HIV associated epigenetic trends and chronic diseases: insights into the hidden burden of chronic infection
Clinical Epigenetics (2026)
-
Neonatal adiposity is associated with microRNAs in adipocyte-derived extracellular vesicles in maternal and cord blood, a discovery analysis
International Journal of Obesity (2024)
-
DNA methylation patterns at birth predict health outcomes in young adults born very low birthweight
Clinical Epigenetics (2023)
-
DNA methylation-based predictors of health: applications and statistical considerations
Nature Reviews Genetics (2022)