Abstract
Background
Obesity is a worldwide problem. Some studies revealed that it leads to deterioration of the cognitive function, regardless of age.
Aim of the study
explore the effect of obesity on cognitive function in a rat model of obesity highlighting the role of glial cells.
Materials and methods
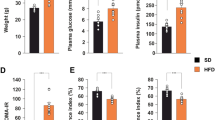
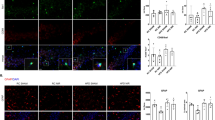
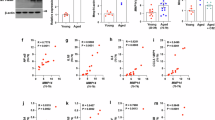
twenty adult male albino rats were assigned to two groups: group I: consumed normal diet, group II: consumed high-fat diet. Body Mass Index (BMI), serum glucose, insulin, HOMA IR and lipid profile were measured. Also, hippocampal expression of Brain derived neurotrophic factor (Bdnf), synapsin, Ionized calcium binding adaptor molecule 1 (Iba), nuclear factor erythroid -related factor 2 (Nrf2), Myelin basic protein (Mbp) were measured by real-time polymerase chain reaction. The Morris Water Maze is a test used to assess spatial learning and memory capacities of rats.
Results
There was a high significant increase in lipid profile, serum glucose, insulin serum levels and HOMA-IR in obese groups with impaired Morris water maze performance compared to control group. There was a significant downregulation in hippocampal Bdnf and synapsin mRNA expression. In addition to decrease in Mbp mRNA expression (P < 0.001). This could be explained by oxidative stress through significant downregulation of Nrf2 mRNA, and inflammation observed in significant upregulation Iba mRNA gene expression in the obese group.
Conclusion
Many factors contribute to obesity associated cognitive impairment. In our study, we figured out the crucial roles of glial cells including microglial activation and oligodendrocytes affection with other underlying mechanisms including oxidative stress and hippocampal inflammation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Castanon N, Lasselin J, Capuron L. Neuropsychiatric comorbidity in obesity: role of inflammatory processes. Front Endocrinol (Lausanne). 2014;5:74 https://doi.org/10.3389/fendo.2014.00074.
Driscoll I, Beydoun MA, An Y, Davatzikos C, Ferrucci L, Zonderman AB, et al. Midlife obesity and trajectories of brain volume changes in older adults. Hum Brain Mapp. 2012;33:2204–10. https://doi.org/10.1002/hbm.21353.
Ronan L, Alexander-Bloch A, Fletcher PC. Childhood obesity, cortical structure, and executive function in healthy children. Cereb Cortex. 2020;30:2519–28. https://doi.org/10.1093/cercor/bhz257.
Jack CR, Petersen RC, Xu YC, O’Brien PC, Smith GE, Ivnik RJ, et al. Prediction of AD with MRI-based hippocampal volume in mild cognitive impairment. Neurology. 1999;52:1397–403. https://doi.org/10.1212/wnl.52.7.1397.
Park DC, Huang CM. Culture wires the Brain: a cognitive neuroscience perspective. Perspect Psychol Sci. 2010;5:391–400. https://doi.org/10.1177/1745691610374591.
Valladolid-Acebes I, Stucchi P, Cano V, Fernández-Alfonso MS, Merino B, Gil-Ortega M, et al. High-fat diets impair spatial learning in the radial-arm maze in mice. Neurobiol Learn Mem. 2011;95:80–85. https://doi.org/10.1016/j.nlm.2010.11.007.
Arnold SE, Arvanitakis Z, Macauley-Rambach SL, Koenig AM, Wang HY, Ahima RS, et al. Brain insulin resistance in type 2 diabetes and Alzheimer disease: concepts and conundrums. Nat Rev Neurol. 2018;14:168–81. https://doi.org/10.1038/nrneurol.2017.185.
Sobesky JL, Barrientos RM, De May HS, Thompson BM, Weber MD, Watkins LR, et al. High-fat diet consumption disrupts memory and primes elevations in hippocampal IL-1β, an effect that can be prevented with dietary reversal or IL-1 receptor antagonism. Brain Behav Immun. 2014;42:22–32. https://doi.org/10.1016/j.bbi.2014.06.017.
Chung WS, Welsh CA, Barres BA, Stevens B. Do glia drive synaptic and cognitive impairment in disease? Nat Neurosci. 2015;18:1539–45. https://doi.org/10.1038/nn.4142.
Nguyen PT, Dorman LC, Pan S, Vainchtein ID, Han RT, Nakao-Inoue H, et al. Microglial remodeling of the extracellular matrix promotes synapse plasticity. Cell. 2020;182:388–403.e15. https://doi.org/10.1016/j.cell.2020.05.050.
Lee W, Moon M, Kim GH, Lee HT, Oh SM. Heat stress-induced memory impairment is associated with neuroinflammation in mice. J Neuroinflammation. 2015;12:102 https://doi.org/10.1186/s12974-015-0324-6.
Wadhwa M, Prabhakar A, Ray K, Roy K, Kumari P, Jha PK, et al. Inhibiting the microglia activation improves the spatial memory and adult neurogenesis in rat hippocampus during 48h of sleep deprivation. J Neuroinflammation. 2017;14:222 https://doi.org/10.1186/s12974-017-0998-z.
Ohsawa K, Imai Y, Kanazawa H, Sasaki Y, Kohsaka S. Involvement of Iba1 in membrane ruffling and phagocytosis of macrophages/microglia. J Cell Sci. 2000;113:3073–84.
Yang C, Tang J, Liang X, Qi Y, Luo Y, Xie Y et al. Anti-LINGO-1 antibody treatment improves chronic stress-induced spatial memory impairments and oligodendrocyte loss in the hippocampus. Behav Brain Res. 2020,393. https://doi.org/10.1016/j.bbr.2020.112765.
Yoon H, Kleven A, Paulsen A, Kleppe L, Wu J, Ying Z, et al. Interplay between exercise and dietary fat modulates myelinogenesis in the central nervous system. Biochim Biophys Acta—Mol Basis Dis. 2016;1862:545–55. https://doi.org/10.1016/j.bbadis.2016.01.019.
Aggarwal S, Yurlova L, Simons M. Central nervous system myelin: structure, synthesis and assembly. Trends Cell Biol. 2011;21:585–93. https://doi.org/10.1016/j.tcb.2011.06.004.
Jovanovic JN, Czernik AJ, Fienberg AA, Greengard P, Sihra TS. Synapsins as mediators of BDNF-enhanced neurotransmitter release. Nat Neurosci. 2000;3:323–9. https://doi.org/10.1038/73888.
Brandes MS, Gray NE NRF2 as a Therapeutic target in neurodegenerative diseases. ASN Neuro. 2020; 12. https://doi.org/10.1177/1759091419899782.
Noeman SA, Hamooda HE, Baalash AA. Biochemical study of oxidative stress markers in the liver, kidney and heart of high fat diet induced obesity in rats. Diabetol Metab Syndr. 2011;3:17 https://doi.org/10.1186/1758-5996-3-17.
Novelli ELB, Diniz YS, Galhardi CM, Ebaid GMX, Rodrigues HG, Mani F, et al. Anthropometrical parameters and markers of obesity in rats. Lab Anim. 2007;41:111–9. https://doi.org/10.1258/002367707779399518.
Sun G, Bishop J, Khalili S, Vasdev S, Gill V, Pace D, et al. Serum visfatin concentrations are positively correlated with serum triacylglycerols and downregulated by overfeeding in healthy young men. Am J Clin Nutr. 2007;85:399–404. https://doi.org/10.1093/ajcn/85.2.399.
Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502.
Silva RH, Abílio VC, Takatsu AL, Kameda SR, Grassl C, Chehin AB, et al. Role of hippocampal oxidative stress in memory deficits induced by sleep deprivation in mice. Neuropharmacology. 2004;46:895–903. https://doi.org/10.1016/j.neuropharm.2003.11.032.
Vorhees CV, Williams MT. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc. 2006;1:848–58. https://doi.org/10.1038/nprot.2006.116.
Bauer CCC, Moreno B, González-Santos L, Concha L, Barquera S, Barrios FA. Child overweight and obesity are associated with reduced executive cognitive performance and brain alterations: A magnetic resonance imaging study in Mexican children. Pediatr Obes. 2015;10:196–204. https://doi.org/10.1111/ijpo.241.
Cournot M, Marquié JC, Ansiau D, Martinaud C, Fonds H, Ferrières J, et al. Relation between body mass index and cognitive function in healthy middle-aged men and women. Neurology. 2006;67:1208–14. https://doi.org/10.1212/01.wnl.0000238082.13860.50.
Ricciarelli R, Canepa E, Marengo B, Marinari UM, Poli G, Pronzato MA, et al. Cholesterol and Alzheimer’s disease: A still poorly understood correlation. IUBMB Life. 2012;64:931–5. https://doi.org/10.1002/iub.1091.
Xue-Shan Z, juan P, Qi W, Zhong R, Li-hong P, Zhi-han T, et al. Imbalanced cholesterol metabolism in Alzheimer’s disease. Clin Chim Acta. 2016;456:107–14. https://doi.org/10.1016/j.cca.2016.02.024.
Uppin V, Acharya P, Bettadaiah Bheemanakere K, Talahalli RR Hyperlipidemia downregulate brain antioxidant defense enzymes and neurotrophins in rats: assessment of the modulatory potential of EPA+DHA and zerumbone. Mol Nutr Food Res. 2020; 64. https://doi.org/10.1002/mnfr.202000381.
Farr SA, Yamada KA, Butterfield DA, Abdul HM, Xu L, Miller NE, et al. Obesity and hypertriglyceridemia produce cognitive impairment. Endocrinology. 2008;149:2628–36. https://doi.org/10.1210/en.2007-1722.
Lee CC, Huang CC. Hsu K Sen. Insulin promotes dendritic spine and synapse formation by the PI3K/Akt/mTOR and Rac1 signaling pathways. Neuropharmacology. 2011;61:867–79. https://doi.org/10.1016/j.neuropharm.2011.06.003.
Zhao F, Siu JJ, Huang W, Askwith C, Cao L. Insulin modulates excitatory synaptic transmission and synaptic plasticity in the mouse hippocampus. Neuroscience. 2019;411:237–54. https://doi.org/10.1016/j.neuroscience.2019.05.033.
Park HS, Park SS, Kim CJ, Kim TW, Kim TW. Exercise alleviates cognitive functions by enhancing hippocampal insulin signaling and neuroplasticity in high-fat diet-induced obesity. Nutrients. 2019;11:1603 https://doi.org/10.3390/nu11071603.
Arvanitakis Z, Wang HY, Capuano AW, Khan A, Taïb B, Anokye-Danso F, et al. Brain insulin signaling, alzheimer disease pathology, and cognitive function. Ann Neurol. 2020;88:513–25. https://doi.org/10.1002/ana.25826.
Chunchai T, Thunapong W, Yasom S, Wanchai K, Eaimworawuthikul S, Metzler G, et al. Decreased microglial activation through gut-brain axis by prebiotics, probiotics, or synbiotics effectively restored cognitive function in obese-insulin resistant rats. J Neuroinflammation. 2018;15:11 https://doi.org/10.1186/s12974-018-1055-2.
Lewis AR, Singh S, Youssef FF. Cafeteria-diet induced obesity results in impaired cognitive functioning in a rodent model. Heliyon. 2019;5:e01412 https://doi.org/10.1016/j.heliyon.2019.e01412.
Holt LM, Hernandez RD, Pacheco NL, Torres Ceja B, Hossain M, Olsen ML. Astrocyte morphogenesis is dependent on BDNF signaling via astrocytic TrkB.T1. Elife. 2019;8:e44667 https://doi.org/10.7554/eLife.44667.
Cunha C, Brambilla R, Thomas KL A simple role for BDNF in learning and memory? Front Mol Neurosci. 2010; 3. https://doi.org/10.3389/neuro.02.001.2010.
Molteni R, Barnard RJ, Ying Z, Roberts CK, Gómez-Pinilla F. A high-fat, refined sugar diet reduces hippocampal brain-derived neurotrophic factor, neuronal plasticity, and learning. Neuroscience. 2002;112:803–14. https://doi.org/10.1016/S0306-4522(02)00123-9.
Fields RD, Bukalo O. Myelin makes memories. Nat Neurosci. 2020;23:469–70.
Jabri MA, Sakly M, Marzouki L, Sebai H. Chamomile (Matricaria recutita L.) decoction extract inhibits in vitro intestinal glucose absorption and attenuates high fat diet-induced lipotoxicity and oxidative stress. Biomed Pharmacother. 2017;87:153–9. https://doi.org/10.1016/j.biopha.2016.12.043.
Morrison CD, Pistell PJ, Ingram DK, Johnson WD, Liu Y, Fernandez-Kim SO, et al. High fat diet increases hippocampal oxidative stress and cognitive impairment in aged mice: implications for decreased Nrf2 signaling. J Neurochem. 2010;114:1581–9. https://doi.org/10.1111/j.1471-4159.2010.06865.x.
Johnson JA, Johnson DA, Kraft AD, Calkins MJ, Jakel RJ, Vargas MR et al. The Nrf2-ARE pathway: An indicator and modulator of oxidative stress in neurodegeneration. In: Annals of the New York Academy of Sciences. 2008; 61–69. https://doi.org/10.1196/annals.1427.036.
Pepping JK, Freeman LR, Gupta S, Keller JN, Bruce-Keller AJ. NOX2 deficiency attenuates markers of adiposopathy and brain injury induced by high-fat diet. Am J Physiol—Endocrinol Metab. 2013;304:E392–E404. https://doi.org/10.1152/ajpendo.00398.2012.
Miller AA, Spencer SJ. Obesity and neuroinflammation: a pathway to cognitive impairment. Brain Behav Immun. 2014;42:10–21. https://doi.org/10.1016/j.bbi.2014.04.001.
Mi Y, Qi G, Fan R, Qiao Q, Sun Y, Gao Y, et al. EGCG ameliorates high-fat- and high-fructose-induced cognitive defects by regulating the IRS/AKT and ERK/CREB/BDNF signaling pathways in the CNS. FASEB J. 2017;31:4998–5011. https://doi.org/10.1096/fj.201700400RR.
Beilharz JE, Maniam J, Morris MJ. Short exposure to a diet rich in both fat and sugar or sugar alone impairs place, but not object recognition memory in rats. Brain Behav Immun. 2014;37:134–41. https://doi.org/10.1016/j.bbi.2013.11.016.
Pipatpiboon N, Pintana H, Pratchayasakul W, Chattipakorn N, Chattipakorn SC. DPP4-inhibitor improves neuronal insulin receptor function, Brain mitochondrial function and cognitive function in rats with insulin resistance induced by high-fat diet consumption. Eur J Neurosci. 2013;37:839–49. https://doi.org/10.1111/ejn.12088.
Jeon BT, Jeong EA, Shin HJ, Lee Y, Lee DH, Kim HJ, et al. Resveratrol attenuates obesity-associated peripheral and central inflammation and improves memory deficit in mice fed a high-fat diet. Diabetes. 2012;61:1444–54. https://doi.org/10.2337/db11-1498.
Kang DH, Heo RW, Yi COK, Kim H, Choi CH, Roh GS. High-fat diet-induced obesity exacerbates kainic acid-induced hippocampal cell death. BMC Neurosci. 2015;16:72 https://doi.org/10.1186/s12868-015-0202-2.
Lizarbe B, Cherix A, Duarte JMN, Cardinaux JR, Gruetter R. High-fat diet consumption alters energy metabolism in the mouse hypothalamus. Int J Obes. 2019;43:1295–304. https://doi.org/10.1038/s41366-018-0224-9.
López-Valdés HE, Martínez-Coria H. The role of neuroinflammation in age-related dementias. Rev Invest Clin. 2016;68:40–48.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wahid, R.M., Samy, W. & El-sayed, S.F. Cognitive impairment in obese rat model: role of glial cells. Int J Obes 45, 2191–2196 (2021). https://doi.org/10.1038/s41366-021-00880-9
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41366-021-00880-9
This article is cited by
-
Investigating the shared genetic architecture between schizophrenia and body mass index
Molecular Psychiatry (2023)