Abstract
Background
Glucagon-like peptide 1 receptor agonists have been proven to be effective in adults with obesity. However, robust evidence on their effects on body weight, obesity-related metabolic changes, and safety in children and adolescents with obesity remains limited, making them a subpopulation with scant treatment options. Therefore, this meta-analysis aimed to determine more precise estimates of the efficacy and safety of glucagon-like peptide-1 agonists in pediatric obesity.
Methods
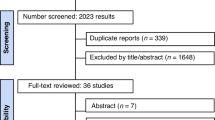
Three databases were searched (PubMed, Embase, and Cochrane Central Register of Controlled Trials) for trials published until the half of September 2024. The search indexing terms included 3 categories: [1] obesity [2], youth, and [3] glucagon-like peptide-1 receptor agonist (GLP-1 RA). Randomized controlled trials in youth with obesity (age ≤ 18 years) that assessed anthropometric and metabolic parameters were included. A total of 2016 studies were retrieved, and 24 full-text articles were screened. The data were analyzed using both mean differences (MDs) and standardized mean differences (SMDs) with 95% CIs and odds ratios (ORs) with 95% CIs. We applied a random effects model. Our outcomes were body weight (BW), BMI, waist circumference (WC), lipid profile, Hb1Ac, fasting blood glucose (FBG), blood pressure, and side effects.
Results
Eight studies comprised of 715 children and adolescents were included. On average, GLP-1 RA reduced BMI (SMD −0.67; 95% CI −0.8 to −0.41), BW (SMD −0.60; 95% CI −0.89 to −0.44), and WC (SMD −0.40; 95% CI −0.61 to −0.18). Although lipid profiles, HbA1c, and FBG were unaffected, GLP-1 RA was linked to a slight reduction in SBP (SMD −0.20; 95% CI −0.35 to −0.04) and an increase in HR (SMD + 0.26; 95% CI + 0.07 to +0.46), with no significant effect on DBP. Adverse effects, primarily nausea and vomiting, were more common in the intervention group, although trial withdrawal rates remained low.
Conclusions
Within this specific population, GLP-1 RAs exhibit significant reductions in BW, BMI, WC, and SBP. The analyses of lipid profiles, DBP, HbA1c, and FBG showed no significant changes. Also, the administration of these medications is concurrent with an elevated incidence of side effects, which are predominantly gastrointestinal and tolerable.
Trial registration
PROSPERO identifier: CRD42024532845.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Lobstein T, Brinsden H, Neveux M. World Obesity Atlas 2022. World Obesity Federation. 2022. [Last accessed on 06 May 2024]. Available from: https://s3-eu-west-1.amazonaws.com/wof-files/World_Obesity_Atlas_2022.pdf
Lange SJ, Kompaniyets L, Freedman DS, Kraus EM, Porter R, Blanck HM, et al. Longitudinal Trends in Body Mass Index Before and During the COVID-19 Pandemic Among Persons Aged 2-19 Years - United States, 2018-2020. MMWR Morb Mortal Wkly Rep. 2021;70:1278–83.
Hampl SE, Hassink SG, Skinner AC, Armstrong SC, Barlow SE, Bolling CF, et al. Clinical practice guideline for the evaluation and treatment of children and adolescents with obesity. Pediatrics. 2023;151:e2022060640.
Kelly AS, Barlow SE, Rao G, Inge TH, Hayman LL, Steinberger J, et al. Severe obesity in children and adolescents: Identification, associated health risks, and treatment approaches: A scientific statement from the American Heart Association. Circulation. 2013;128(Oct):1689–712.
Cercato C, Fonseca FA. Cardiovascular risk and obesity. Diabetol Metab Syndr. 2019;11:1–15.
Salam RA, Padhani ZA, Das JK, Shaikh AY, Hoodbhoy Z, Jeelani SM, et al. Effects of lifestyle modification interventions to prevent and manage child and adolescent obesity: a systematic review and meta-analysis. Nutrients. 2020;12:1–23.
Garvey WT. Is obesity or adiposity-based chronic disease curable: the set point theory, the environment, and second-generation medications. Endocr Pract. 2022;28:214–22.
Grossman DC, Bibbins-Domingo K, Curry SJ, Barry MJ, Davidson KW, Doubeni CA, et al. Screening for obesity in children and adolescents: us preventive services task force recommendation statement. JAMA. 2017;317:2417–26.
Holst JJ. GLP-1 physiology in obesity and development of incretin-based drugs for chronic weight management. Nat Metab. 2024;6:1866–85.
Iqbal J, Wu HX, Hu N, Zhou YH, Li L, Xiao F, et al. Effect of glucagon-like peptide-1 receptor agonists on body weight in adults with obesity without diabetes mellitus—a systematic review and meta-analysis of randomized control trials. Obes Rev. 2022;23:e13435.
Kelly AS, Auerbach P, Barrientos-Perez M, Gies I, Hale PM, Marcus C, et al. A randomized, controlled trial of liraglutide for adolescents with obesity. N Engl J Med. 2020;382:2117–28.
Shah AS, Zeitler PS, Wong J, Pena AS, Wicklow B, Arslanian S, et al. ISPAD Clinical Practice Consensus Guidelines 2022: Type 2 diabetes in children and adolescents. Pediatr Diabetes. 2022;23:872–902.
Weghuber D, Barrett T, Barrientos-Pérez M, Gies I, Hesse D, Jeppesen OK, et al. Once-weekly semaglutide in adolescents with obesity. N Engl J Med. 2022;387:2245–57.
Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898.
Schünemann H, Brożek J, Guyatt G, Oxman A, editors. GRADE handbook for grading quality of evidence and strength of recommendations. Updated October 2013. The GRADE Working Group, 2013. Available from guidelinedevelopment.org/handbook.
GRADEpro GDT: GRADEpro Guideline Development Tool [Software]. McMaster University and Evidence Prime, 2024. Available from gradepro.org.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
Danne T, Biester T, Kapitzke K, Jacobsen SH, Jacobsen LV, Petri KCC, et al. Liraglutide in an adolescent population with obesity: a randomized, double-blind, placebo-controlled 5-week trial to assess safety, tolerability, and pharmacokinetics of liraglutide in adolescents aged 12–17 Years. J Pediatr. 2017;181:146–53.e3.
Mastrandrea LD, Witten L, Carlsson Petri KC, Hale PM, Hedman HK, Riesenberg RA. Liraglutide effects in a paediatric (7-11 y) population with obesity: A randomized, double-blind, placebo-controlled, short-term trial to assess safety, tolerability, pharmacokinetics, and pharmacodynamics. Pediatr Obes. 2019;14:e12495.
Weghuber D, Forslund A, Ahlström H, Alderborn A, Bergström K, Brunner S, et al. A 6-month randomized, double-blind, placebo-controlled trial of weekly exenatide in adolescents with obesity. Pediatr Obes. 2020;15:e12624.
Fox CK, Clark JM, Rudser KD, Ryder JR, Gross AC, Nathan BM, et al. Exenatide for weight-loss maintenance in adolescents with severe obesity: A randomized, placebo-controlled trial. Obesity. 2022;30:1105–15.
Kelly AS, Rudser KD, Nathan BM, Fox CK, Metzig AM, Coombes BJ, et al. The effect of Glucagon-like peptide-1 receptor agonist therapy on body mass index in adolescents with severe obesity. JAMA Pediatr. 2013;167:355–60.
Fox CK, Barrientos-Pérez M, Bomberg EM, Dcruz J, Gies I, Harder-Lauridsen NM, et al. Liraglutide for Children 6 to <12 Years of age with obesity — a randomized trial. N Engl J Med. 2024;392:555–65.
Chadda KR, Cheng TS, Ong KK. GLP-1 agonists for obesity and type 2 diabetes in children: Systematic review and meta-analysis. Obes Rev. 2021;22:e13177.
Ryan PM, Seltzer S, Hayward NE, Rodriguez DA, Sless RT, Hawkes CP. Safety and efficacy of glucagon-like peptide-1 receptor agonists in children and adolescents with obesity: a meta-analysis. J Pediatr. 2021;236:137–47.e13.
Wahi G, St-Pierre J, Johnston BC, Fitzpatrick-Lewis D, Usman A, Sherifali D, et al. Effectiveness of pharmacological interventions for managing obesity in children and adolescents: A systematic review and meta-analysis framed using minimal important difference estimates based on GRADE guidance to inform a clinical practice guideline. Pediatr Obes. 2024;19:e13169.
Liu L, Shi H, Shi Y, Wang A, Guo N, Tao H, et al. Comparative Efficacy and Safety of Glucagon-like Peptide-1 Receptor Agonists in Children and Adolescents with Obesity or Overweight: A Systematic Review and Network Meta-Analysis. Pharmaceuticals. 2024;17:828.
Alkhezi OS, Alahmed AA, Alfayez OM, Alzuman OA, Almutairi AR, Almohammed OA. Comparative effectiveness of glucagon-like peptide-1 receptor agonists for the management of obesity in adults without diabetes: A network meta-analysis of randomized clinical trials. Obes Rev. 2023;24:e13543.
Bunck MC, Diamant M, Eliasson B, Cornér A, Shaginian RM, Heine RJ, et al. Exenatide Affects Circulating Cardiovascular Risk Biomarkers Independently of Changes in Body Composition. Diabetes Care. 2010;33:1734–7.
Kosiborod MN, Bhatta M, Davies M, Deanfield JE, Garvey WT, Khalid U, et al. Semaglutide improves cardiometabolic risk factors in adults with overweight or obesity: STEP 1 and 4 exploratory analyses. Diabetes, Obes Metab. 2023;25:468–78.
Yugar LBT, Sedenho-Prado LG, da Silva Ferreira IMC, Silva CAM, Sposito AC, Cercato C. The efficacy and safety of GLP-1 receptor agonists in youth with type 2 diabetes: a meta-analysis. Diabetol Metab Syndr. 2024;16:92.
Arastu N, Cummins O, Uribe W, Nemec EC. Efficacy of subcutaneous semaglutide compared to placebo for weight loss in obese, non-diabetic adults: a systematic review & meta-analysis. Int J Clin Pharm. 2022;44:852–9.
Vilsbøll T, Christensen M, Junker AE, Knop FK, Gluud LL. Effects of glucagon-like peptide-1 receptor agonists on weight loss: systematic review and meta-analyses of randomised controlled trials. BMJ. 2012;344:d7771.
Ogden CL, Fryar CD, Martin CB, Freedman DS, Carroll MD, Gu Q, et al. Trends in Obesity Prevalence by Race and Hispanic Origin—1999-2000 to 2017–2018. JAMA. 2020;324:1208–10.
Acknowledgements
To conduct this meta-analysis, the first author (L.G.S.P) received a research scholarship granted by the Brazilian National Research Council (CNPq) under the protocol 134326/2024-5.
Funding
Prof. Sposito was supported by a Research Career Awards grant from the Brazilian National Research Council (CNPq) (grant number 304257/2021-4). Cintia Cercato declares having received consulting honoraria from Novo Nordisk, Eli Lilly, Merck, Brace Pharma and Eurofarma.
Author information
Authors and Affiliations
Contributions
LGSP, LBTY, IMCSF, ARW, MPM, and DCJ collected the clinical trial data. CAMS contributed to the statistical analysis of the collected data. LGSP and LBTY drafted the manuscript. ACS and CC reviewed the final manuscript for critical content, had full access to all study data and took responsibility for data integrity and analytical accuracy.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sedenho-Prado, L.G., Yugar, L.B.T., Whitaker, A.R. et al. Metabolic outcomes and safety of GLP-1 receptor agonists in children and adolescents with obesity: A systematic review and meta-analysis. Int J Obes 49, 1469–1479 (2025). https://doi.org/10.1038/s41366-025-01790-w
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41366-025-01790-w