Abstract
Background
Maternal nutrition during critical developmental windows is increasingly recognised as a key determinant of offspring health, a concept central to the developmental origins of health and disease paradigm. Monosodium glutamate (MSG), a common food additive and neuroendocrine stimulant, warrants investigation in this context.
Methods
The impact of maternal MSG exposure (120 mg/kg) during gestation and/or lactation on metabolic programming in first-generation male rat offspring. Metabolic, hormonal, and molecular parameters in pups following maternal MSG administration, body weight, food intake, adiposity, glucose homeostasis (insulin sensitivity and resistance), lipid profiles, oxidative stress markers, and inflammatory mediators were measured. Furthermore, we analyzed microRNA expression profiles in relevant tissues.
Results
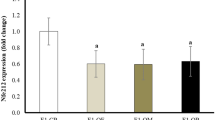
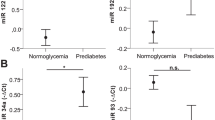
Maternal MSG exposure resulted in significant metabolic perturbations in offspring. Key findings included a reduced survival index, impaired glucose homeostasis (manifesting as decreased insulin sensitivity and increased insulin resistance), increased body weight, and elevated adiposity. We observed elevated oxidative stress, dyslipidemia, altered lipid peroxidation, and hormonal imbalances. Quantitative PCR analysis revealed altered expression of metabolic and inflammatory genes in both adipocytes and the hypothalamus. MicroRNA expression analysis identified significant alterations in miR-27a, miR-34a, miR-335, and miR-30a, suggesting potential regulatory roles in adipogenesis and metabolic control.
Conclusions
Maternal MSG exposure during gestation and lactation induces profound and adverse metabolic effects in male offspring. The observed alterations in metabolic indices suggest an increased risk of metabolic disease later in life. This study underscores the critical importance of maternal nutrition during sensitive developmental periods and has implications for public health recommendations and nutritional guidelines (Graphical abstract).

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout














Similar content being viewed by others
Data availability
Data are available upon reasonable request to the corresponding author.
References
Soltani Z, Shariatpanahi M, Aghsami M, Owliaey H, Kheradmand A. Investigating the effect of exposure to monosodium glutamate during pregnancy on development of autism in male rat offspring. Food Chem Toxicol. 2024;185:114464.
Kayode OT, Bello JA, Oguntola JA, Kayode AAA, Olukoya DK. The interplay between monosodium glutamate (MSG) consumption and metabolic disorders. Heliyon. 2023;9:e19675.
Van Winkle LJ. Perspective: might maternal dietary monosodium glutamate (MSG) consumption impact pre- and peri-implantation embryos and their subsequent development? Int J Environ Res Public Health. 2022;19:13611.
Gowda S, Seibert T, Uli N, Farrell R. Pediatric obesity: endocrinologic and genetic etiologies and management. Curr Cardiovasc Risk Rep. 2019;13:39.
Yadav HM, Jawahar A. Environmental factors and obesity. StatPearls, 2022.
Mishra G, Townsend KL. The metabolic and functional roles of sensory nerves in adipose tissues. Nat Metab. 2023;5:1461–74.
Liu Z, Xiao T, Liu H. Leptin signaling and its central role in energy homeostasis. Front Neurosci. 2023;17. https://doi.org/10.3389/fnins.2023.1238528.
Boucsein A, Kamstra K, Tups A. Central signalling cross-talk between insulin and leptin in glucose and energy homeostasis. J Neuroendocrinol. 2021;33. https://doi.org/10.1111/jne.12944.
Dearden L, Buller S, Furigo IC, Fernandez-Twinn DS, Ozanne SE. Maternal obesity causes fetal hypothalamic insulin resistance and disrupts development of hypothalamic feeding pathways. Mol Metab. 2020;42:101079.
Al-Otaibi A, Mansour N, Elabd H, Esmail N. Toxicity of monosodium glutamate intake on different tissues induced oxidative stress: a review. J Med Life Sci. 2022;0:68–81.
Phatak S, Janesick AS, Schug TT, Heindel JJ, Blumberg B. Environmental chemicals and obesity. In: Handbook of Obesity – Volume 1. CRC Press, Taylor & Francis; 2024. p. 441–8.
Shang R, Lee S, Senavirathne G, Lai EC. microRNAs in action: biogenesis, function and regulation. Nat Rev Genet. 2023;24:816–33.
Hernández-Gómez KG, Avila-Nava A, González-Salazar LE, Noriega LG, Serralde-Zúñiga AE, Guizar-Heredia R, et al. Modulation of MicroRNAs and exosomal MicroRNAs after dietary interventions for obesity and insulin resistance: a narrative review. Metabolites. 2023;13:1190.
Park J-H, Choi T-S. Subcutaneous administration of monosodium glutamate to pregnant mice reduces weight gain in pups during lactation. Lab Anim. 2016;50:94–99.
Song W, Chen J, Petrilli A, Liot G, Klinglmayr E, Zhou Y, et al. Mutant huntingtin binds the mitochondrial fission GTPase dynamin-related protein-1 and increases its enzymatic activity. Nat Med. 2011;17:377–82.
Olney JW. Brain lesions, obesity, and other disturbances in mice treated with monosodium glutamate. Science ((1979)). 1969;164:719–21.
Panjabud P, Kanlayaprasit S, Thongkorn S, Songsritaya K, Lertpeerapan P, Kasitipradit K, et al. Prenatal exposure to bisphenol A disrupts RNA splicing in the prefrontal cortex and promotes behaviors related to autism in offspring. Sci Rep. 2025;15:25996.
Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur J Pharm. 2003;463:3–33.
Shinohara H, Matsubayashi Y. Analysis of PLETHORA gradient formation by secreted peptides during root development. Morphogen gradients: methods and protocols. Springer, 2018. p. 155–64.
Martins ACP, Borges HE, Garcia RMG, Carniatto SR, Mathias PCF. Monosodium L-glutamate-induced obesity impaired the adrenal medullae activity. Neurosci Res Commun. 2001;28:49–58.
Diniz YS, Faine LA, Almeida JA, Silva MDP, Ribas BO, Novelli ELB. Toxicity of dietary restriction of fat enriched diets on cardiac tissue. Food Chem Toxicol. 2002;40:1893–9.
Wang R, Yin Y, Li J, Wang H, Lv W, Gao Y, et al. Global stable-isotope tracing metabolomics reveals system-wide metabolic alternations in aging Drosophila. Nat Commun. 2022;13:3518.
Yang X, Kou T, Wang H, Zhu J, Zhu Z-J, Cai Y. S-adenosylmethionine metabolism shapes CD8+ T cell functions in colorectal cancer. Cancer Metab. 2025;13:23.
Katz A, Nambi SS, Mather K, Baron AD, Follmann DA, Sullivan G, et al. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab. 2000;85:2402–10.
Yokoyama H, Emoto M, Fujiwara S, Motoyama K, Morioka T, Komatsu M, et al. Quantitative insulin sensitivity check index and the reciprocal index of homeostasis model assessment in normal range weight and moderately obese type 2 diabetic patients. Diab Care. 2003;26:2426–32.
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and ?-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9.
Barham D, Trinder P. An improved colour reagent for the determination of blood glucose by the oxidase system. Analyst. 1972;97:142–5.
Trinder P. Determination of glucose in blood using glucose oxidase with an alternative oxygen acceptor. Ann Clin Biochem. 1969;6:24–27.
Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502.
Owen JB, Butterfield DA. Measurement of oxidized/reduced glutathione ratio. Methods Mol Biol. 2010;643:269–77.
Griffith OW. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal Biochem. 1980;106:207–12.
Beutler E, Gelbart T. Plasma glutathione in health and in patients with malignant disease. J Lab Clin Med. 1985;105:581–4.
VanGuilder HD, Vrana KE, Freeman WM. Twenty-five years of quantitative PCR for gene expression analysis. Biotechniques. 2008;44:619–26.
Dweep H, Gretz N. miRWalk2.0: a comprehensive atlas of microRNA-target interactions. Nat Methods. 2015;12:697–697.
Xie H, Sun L, Lodish HF. Targeting microRNAs in obesity. Expert Opin Ther Targets. 2009;13:1227–38.
Ogata H, Goto S, Sato K, Fujibuchi W, Bono H, Kanehisa M. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 1999;27:29–34.
Biney RP, Djankpa FT, Osei SA, Egbenya DL, Aboagye B, Karikari AA, et al. Effects of in utero exposure to monosodium glutamate on locomotion, anxiety, depression, memory and KCC2 expression in offspring. Int J Dev Neurosci. 2022;82:50–62.
Meex RCR, Blaak EE, van Loon LJC. Lipotoxicity plays a key role in the development of both insulin resistance and muscle atrophy in patients with type 2 diabetes. Obes Rev. 2019;20:1205–17.
Fazakerley DJ, van Gerwen J, Cooke KC, Duan X, Needham EJ, Díaz-Vegas A, et al. Phosphoproteomics reveals rewiring of the insulin signaling network and multi-nodal defects in insulin resistance. Nat Commun. 2023;14:923.
Haddad M, Esmail R, Khazali H. Reporting the effects of exposure to monosodium glutamate on the regulatory peptides of the hypothalamic-pituitary-gonadal axis. Int J Fertil Steril. 2021;15:246–51.
Kesherwani R, Bhoumik S, Kumar R, Rizvi SI. Monosodium glutamate even at low dose may affect oxidative stress, inflammation and neurodegeneration in rats. Indian J Clin Biochem. 2024;39:101–9.
Liang X, Yang Q, Zhang L, Maricelli JW, Rodgers BD, Zhu M-J, et al. Maternal high-fat diet during lactation impairs thermogenic function of brown adipose tissue in offspring mice. Sci Rep. 2016;6:34345.
Kreuz S, Joubert E, Waldmann K-H, Ternes W. Aspalathin, a flavonoid in Aspalathus linearis (rooibos), is absorbed by pig intestine as a C-glycoside. Nutr Res. 2008;28:690–701.
Seow K-M, Lee J-L, Doong M-L, Huang S-W, Hwang J-L, Huang W-J, et al. Human chorionic gonadotropin regulates gastric emptying in ovariectomized rats. J Endocrinol. 2013;216:14.
Wu LL, Russell DL, Wong SL, Chen M, Tsai T-S, St John JC, et al. Mitochondrial dysfunction in oocytes of obese mothers: transmission to offspring and reversal by pharmacological endoplasmic reticulum stress inhibitors. Development. 2015;142:681–91.
Masenga SK, Kabwe LS, Chakulya M, Kirabo A. Mechanisms of oxidative stress in metabolic syndrome. Int J Mol Sci. 2023;24:7898.
Park W, Wei S, Kim B-S, Kim B, Bae S-J, Chae YC, et al. Diversity and complexity of cell death: a historical review. Exp Mol Med. 2023;55:1573–94.
Shende P, Desai D. Physiological and therapeutic roles of neuropeptide Y on biological functions. Cell biology and translational medicine, volume 7: stem cells and therapy: emerging approaches. Springer, 2020. p. 37–47.
Park C, Kim J, Ko S-B, Choi YK, Jeong H, Woo H, et al. Structural basis of neuropeptide Y signaling through Y1 receptor. Nat Commun. 2022;13:853.
Banerjee A, Mukherjee S, Maji BK. Worldwide flavor enhancer monosodium glutamate combined with high lipid diet provokes metabolic alterations and systemic anomalies: an overview. Toxicol Rep. 2021;8:938–61.
Abo Zeid AA, Rowida Raafat I, Ahmed AG. Berberine alleviates monosodium glutamate induced postnatal metabolic disorders associated vascular endothelial dysfunction in newborn rats: possible role of matrix metalloproteinase-1. Arch Physiol Biochem. 2022;128:818–29.
Cho YK, Lee S, Lee J, Doh J, Park J-H, Jung Y-S, et al. Lipid remodeling of adipose tissue in metabolic health and disease. Exp Mol Med. 2023;55:1955–73.
Su Y, Feng Z, He Y, Hong L, Liu G, Li T, et al. Monosodium L-glutamate and fats change free fatty acid concentrations in intestinal contents and affect free fatty acid receptors express profile in growing pigs. Food Nutr Res. 2019;63. https://doi.org/10.29219/fnr.v63.1444.
Ghaffari-Nasab A, Javani G, Farajdokht F, Alipour MR, Mohaddes G. Chronic stress-induced anxiety-like behavior, hippocampal oxidative, and endoplasmic reticulum stress are reversed by young plasma transfusion in aged adult rats. Iran J Basic Med Sci. 2024;27:114.
Inzani I, Ozanne SE. Programming by maternal obesity: a pathway to poor cardiometabolic health in the offspring. Proc Nutr Soc. 2022;81:227–42.
Scheidl TB, Brightwell AL, Easson SH, Thompson JA. Maternal obesity and programming of metabolic syndrome in the offspring: searching for mechanisms in the adipocyte progenitor pool. BMC Med. 2023;21:50.
Grilo LF, Diniz MS, Tocantins C, Areia AL, Pereira SP. The endocrine–metabolic axis regulation in offspring exposed to maternal obesity—cause or consequence in metabolic disease programming? Obesities. 2022;2:236–55.
Beam A, Clinger E, Hao L. Effect of diet and dietary components on the composition of the gut microbiota. Nutrients. 2021;13:2795.
María Catalina O, Marta Delia P, Gilda Celina R, Darío M, Verónica L, María Rosa V. Monosodium glutamate affects metabolic syndrome risk factors on obese adult rats: a preliminary study. J Obes Weight Loss Medicat. 2018;4. https://doi.org/10.23937/2572-4010.1510023.
Nauck MA, Quast DR, Wefers J, Meier JJ. GLP-1 receptor agonists in the treatment of type 2 diabetes–state-of-the-art. Mol Metab. 2021;46:101102.
Armstrong J, Hickey G, Diekhans M, Fiddes IT, Novak AM, Deran A, et al. Progressive cactus is a multiple-genome aligner for the thousand-genome era. Nature. 2020;587:246–51.
Kurylowicz A. microRNAs in human adipose tissue physiology and dysfunction. Cells. 2021;10:3342.
Saha PK, Hamilton MP, Rajapakshe K, Putluri V, Felix JB, Masschelin P, et al. miR-30a targets gene networks that promote browning of human and mouse adipocytes. Am J Physiol-Endocrinol Metab. 2020;319:E667–E677.
Martinez Calejman C, Trefely S, Entwisle SW, Luciano A, Jung SM, Hsiao W, et al. mTORC2-AKT signaling to ATP-citrate lyase drives brown adipogenesis and de novo lipogenesis. Nat Commun. 2020;11:575.
Jiang X, Liu K, Luo P, Li Z, Xiao F, Jiang H, et al. Hypothalamic SLC7A14 accounts for aging-reduced lipolysis in white adipose tissue of male mice. Nat Commun. 2024;15:7948.
Numan M, Stolzenberg DS. Medial preoptic area interactions with dopamine neural systems in the control of the onset and maintenance of maternal behavior in rats. Front Neuroendocrinol. 2009;30:46–64.
Champagne FA, Meaney MJ. Transgenerational effects of social environment on variations in maternal care and behavioral response to novelty. Behav Neurosci. 2007;121:1353.
Acknowledgements
The authors extend their appreciation to the Deanship of Research and Graduate Studies at King Khalid University for funding this work through Large Research Project under grant number RGP2/536/46.
Funding
This work was funded by the Deanship of Research and Graduate Studies at King Khalid University through Large Research Project under grant number RGP2/536/46.
Author information
Authors and Affiliations
Contributions
HHA contributed to the conceptualization, supervision, and funding acquisition. EMEN contributed to the methodology, investigation, and data analysis. NSA-Z contributed to the investigation and resources. HZ contributed to the investigation and resources. HEH contributed to the conceptualization, methodology, investigation, data analysis, and writing of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The experimental protocol was approved by the Ethical Committee of the Atomic Energy Authority, Egypt (Approval No. 85-23). All methods were performed in accordance with the relevant guidelines and regulations and complied with the “Guide for the Care and Use of Laboratory Animals” published by the U.S. National Institutes of Health (NIH Publication No. 85-23, 1996).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hassan, A.H., El Nashar, E.M., Al-Zahrani, N.S. et al. Maternal monosodium glutamate exposure disrupts leptin and insulin signaling in the hypothalamus, activating NF-κB and mTOR inflammatory pathways, contributing to metabolic dysfunction in male offspring. Int J Obes 50, 238–259 (2026). https://doi.org/10.1038/s41366-025-01941-z
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41366-025-01941-z