Abstract
Background
This study aims to assess the impact of insulin resistance (IR) on gut microbiota (GM) composition, incretin responses, and metabolic outcomes following sleeve gastrectomy (SG) in people with severe obesity who do not have diabetes.
Methods
A prospective single-center study encompassed patients with severe obesity and normal glucose tolerance who underwent SG. Participants were stratified into two cohorts based on the magnitude of their insulin resistance state, as determined by the Homeostatic Model Assessment of Insulin Resistance (HOMA-IR) index: high-IR (Hi-IR; HOMA-IR > 95th percentile) and low-IR (Lo-IR; HOMA-IR <25th percentile). Body composition measurements, biochemical analyses, and microbiota assessments were performed before and 6 months post-surgery. Additionally, the responses to a standardized meal tolerance test (MTT) of glucagon-like peptide-1 (GLP-1) and glucagon-like peptide-2 (GLP-2) were evaluated.
Results
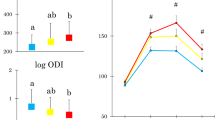
The study cohort consisted of 18 patients (9 with Hi-IR and 9 with Lo-IR), with a mean age of 48.8 ± 9.2 years and a mean body mass index (BMI) of 45.03 ± 4.82 kg/m². Six months post-surgery, the mean percentage of total weight loss (WL) was 26.5 ± 6%, with both groups exhibiting enhanced secretion of GLP-1 and GLP-2 following MTT. At baseline, participants exhibited distinct microbiota profiles; the Hi-IR group showed a higher relative abundance of Prevotella species, which are previously associated with adverse metabolic and inflammatory profiles. Post-surgery, both groups exhibited positive incretin responses and significant modifications in GM composition. Notably, Hi-IR people experienced more pronounced changes in microbial diversity, including increases in Akkermansia and Veillonella species and decreases in Prevotella species. Enhanced GLP-1 and GLP-2 responses were correlated with WL and metabolic improvement, particularly in the Lo-IR population.
Conclusions
These findings underscore the role of GM in metabolic changes and surgical outcomes after SG. Targeting gut microbiota may offer a promising avenue for improving obesity treatment strategies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The microbiome data presented in this study are available in the European Nucleotide Archive (ENA) at https://www.ebi.ac.uk/ena/browser/view/PRJEB48776, accession number PRJEB48776. The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Castagneto-Gissey L, Mingrone G. Insulin sensitivity and secretion modifications after bariatric surgery. J Endocrinol Investig. 2012;35:692–8. https://doi.org/10.3275/8470.
Kashyap SR, Bhatt DL, Wolski K, Watanabe RM, Abdul-Ghani M, Abood B, et al. Metabolic effects of bariatric surgery in patients with moderate obesity and type 2 diabetes: analysis of a randomized control trial comparing surgery with intensive medical treatment. Diabetes Care. 2013;36:2175–82.
Cornejo-Pareja I, Molina-Vega M, Gómez-Pérez AM, Damas-Fuentes M, Tinahones FJ. Factors related to weight loss maintenance in the medium-long term after bariatric surgery: a review. J Clin Med. 2021;10:1739.
Sabah SA, Haddad EA, Qadhi I, AlMuhaini M, AlAwtan A, AlQabandi OA. et al. Beyond the decade: unveiling long-term weight and co-morbidity outcomes up to 10 years post laparoscopic sleeve gastrectomy. Langenbecks Arch Surg. 2025;410:112.
Seeley RJ, Chambers AP, Sandoval DA. The role of gut adaptation in the potent effects of multiple bariatric surgeries on obesity and diabetes. Cell Metab. 2015;21:369–78.
Salminen P, Helmio M, Ovaska J, Juuti A, Leivonen M, Peromaa-Haavisto P, et al. Effect of laparoscopic sleeve gastrectomy vs laparoscopic Roux-en-Y gastric bypass on weight loss at 5 years among patients with morbid obesity: the SLEEVEPASS randomized clinical trial. JAMA. 2018;319:241–54.
Casella G, Soricelli E, Castagneto-Gissey L, Redler A, Basso N, Mingrone G. Changes in insulin sensitivity and secretion after sleeve gastrectomy. Br J Surg. 2016;103:242–8.
Bradley D, Magkos F, Eagon JC, et al. Matched weight loss induced by sleeve gastrectomy or gastric bypass similarly improves metabolic function in obese subjects. Obesity (Silver Spring). 2014;22:2026–31.
Romero F, Nicolau J, Flores L, Casamitjana R, Ibarzabal A, Lacy A, et al. Comparable early changes in gastrointestinal hormones after sleeve gastrectomy and Roux-En-Y gastric bypass surgery for morbidly obese type 2 diabetic subjects. Surg Endosc. 2012;26:2231–9.
Papamargaritis D, Le Roux CW. Do gut hormones contribute to weight loss and glycaemic outcomes after bariatric surgery?. Nutrients. 2021;13:762.
Wilbrink JA, van Avesaat M, Nienhuijs SW, Stronkhorst A, Masclee AAM. Changes in gastrointestinal motility and gut hormone secretion after Roux-en-Y gastric bypass and sleeve gastrectomy for individuals with severe obesity. Clin Obes. 2025;15:e12721.
Dang JT, Mocanu V, Park H, Laffin M, Hotte N, Karmali S, et al. Roux-en-Y gastric bypass and sleeve gastrectomy induce substantial and persistent changes in microbial communities and metabolic pathways. Gut Microbes. 2022;14:2050636.
Tremaroli V, Karlsson F, Werling M, Ståhlman M, Kovatcheva-Datchary P, Olbers T, et al. Roux-en-Y gastric bypass and vertical banded gastroplasty induce long-term changes on the human gut microbiome contributing to fat mass regulation. Cell Metab. 2015;22:228–38.
Yoon HS, Cho CH, Yun MS, Jang SJ, You HJ, Kim JH, et al. Akkermansia muciniphila secretes a glucagon-like peptide-1-inducing protein that improves glucose homeostasis and ameliorates metabolic disease in mice. Nat Microbiol. 2021;6:563–73.
Rowland I, Gibson G, Heinken A, Scott K, Swann J, Thiele I, et al. Gut microbiota functions: metabolism of nutrients and other food components. Eur J Nutr. 2018;57:1–24.
Anhê FF, Zlitni S, Zhang SY, Choi BSY, Chen CY, Foley KP, et al. Human gut microbiota after bariatric surgery alters intestinal morphology and glucose absorption in mice independently of obesity. Gut. 2023;72:460–71.
Damms-Machado A, Mitra S, Schollenberger AE, Kramer KM, Meile T, Königsrainer A, et al. Effects of surgical and dietary weight loss therapy for obesity on gut microbiota composition and nutrient absorption. Biomed Res Int. 2015;2015:806248.
Haghighat N, Ashtary-Larky D, Bagheri R, Aghakhani L, Asbaghi O, Amini M, et al. Preservation of fat-free mass in the first year after bariatric surgery: a systematic review and meta-analysis of 122 studies and 10,758 participants. Surg Obes Relat Dis. 2022;18:964–82.
Preda A, Carbone F, Tirandi A, Montecucco F, Liberale L. Obesity phenotypes and cardiovascular risk: from pathophysiology to clinical management. Rev Endocr Metab Disord. 2023;24:901–19.
Tanriover C, Copur S, Gaipov A, Ozlusen B, Akcan RE, Kuwabara M, et al. Metabolically healthy obesity: misleading phrase or healthy phenotype?. Eur J Intern Med. 2023;111:5–20.
Hjorth MF, Ritz C, Blaak EE, Saris WH, Langin D, Poulsen SK, et al. Pretreatment fasting plasma glucose and insulin modify dietary weight loss success: results from 3 randomized clinical trials. Am J Clin Nutr. 2017;106:499–505.
American Diabetes Association. 2. Diagnosis and classification of diabetes: Standards of Care in Diabetes—2024. Diabetes Care. 2024;47:S20–S42.
Gayoso-Diz P, Otero-González A, Rodriguez-Alvarez MX, Gude F, García F, De Francisco A, et al. Insulin resistance (HOMA-IR) cut-off values and the metabolic syndrome in a general adult population: effect of gender and age: EPIRCE cross-sectional study. BMC Endocr Disord. 2013;13:47.
Decaro-Fragoso MF, Estrada-Garcia T, Lopez-Saucedo C, Elizalde-Barrera CI. Determining insulin resistance cutoffs in mexican adults: percentile distribution vs. receiver operating characteristic curve analysis. Cureus. 2025;17:e79775.
Ballesteros Pomar MD, Vilarrasa García N, Rubio Herrera MÁ, Barahona MJ, Bueno M, Caixàs A, et al. Abordaje clínico integral SEEN de la obesidad en la edad adulta: resumen ejecutivo. Endocrinol Diabetes Nutr (Engl Ed). 2021;68:130–6.
Menzel P, Ng KL, Krogh A. Fast and sensitive taxonomic classification for metagenomics with Kaiju. Nat Commun. 2016;7:11257.
McMurdie PJ, Holmes S. Phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PLoS One. 2013;8:e61217.
Paulson JN, Stine OC, Bravo HC, Pop M. Differential abundance analysis for microbial marker-gene surveys. Nat Methods. 2013;10:1200–2.
Abdelsalam NA, Hegazy SM, Aziz RK. The curious case of Prevotella copri. Vol. 15. Gut Microbes. 2023;15:2249152.
Pedersen HK, Gudmundsdottir V, Nielsen HB, Hyotylainen T, Nielsen T, Jensen BAH, et al. Human gut microbes impact host serum metabolome and insulin sensitivity. Nature. 2016;535:376–81.
Moreno-Indias I, Sánchez-Alcoholado L, García-Fuentes E, Cardona F, Queipo-Ortuño MI, Tinahones FJ. Insulin resistance is associated with specific gut microbiota in appendix samples from morbidly obese patients. Am J Transl Res. 2016;8:5672–84.
Everard A, Belzer C, Geurts L, Ouwerkerk JP, Druart C, Bindels LB, et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci USA. 2013;110:9066–71.
Hernández-Montoliu L, Rodríguez-Peña MM, Puig R, Astiarraga B, Guerrero-Pérez F, Virgili N, et al. A specific gut microbiota signature is associated with an enhanced GLP-1 and GLP-2 secretion and improved metabolic control in patients with type 2 diabetes after metabolic Roux-en-Y gastric bypass. Front Endocrinol. 2023;14:1181744.
Zhong H, Ren H, Lu Y, Fang C, Hou G, Yang Z, et al. Distinct gut metagenomics and metaproteomics signatures in prediabetics and treatment-naïve type 2 diabetics. EBioMedicine. 2019;47:373–83.
Karlsson FH, Tremaroli V, Nookaew I, Bergström G, Behre CJ, Fagerberg B, et al. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature. 2013;498:99–103.
Allin KH, Tremaroli V, Caesar R, Jensen BAH, Damgaard MTF, Bahl MI, et al. Aberrant intestinal microbiota in individuals with prediabetes. Diabetologia. 2018;61:810–20.
Amato A, Baldassano S, Mulè F. GLP2: an underestimated signal for improving glycaemic control and insulin sensitivity. J Endocrinol. 2016;229:R57–R66.
Sridhar A, Khan D, Elliott JA, Naughton V, Flatt PR, Irwin N, et al. RYGB surgery has modest effects on intestinal morphology and gut hormone populations in the bypassed biliopancreatic limb but causes reciprocal changes in GLP-2 and PYY in the alimentary limb. PLoS One. 2023;18:e0286062.
Ikeda T, Aida M, Yoshida Y, Matsumoto S, Tanaka M, Nakayama J, et al. Alteration in faecal bile acids, gut microbial composition and diversity after laparoscopic sleeve gastrectomy. Br J Surg. 2020;107:1673–85.
Farin W, Oñate FP, Plassais J, Bonny C, Beglinger C, Woelnerhanssen B, et al. Impact of laparoscopic Roux-en-Y gastric bypass and sleeve gastrectomy on gut microbiota: a metagenomic comparative analysis. Surg Obes Relat Dis. 2020;16:852–62.
Morán-Ramos S, Soriano-Cortés R, Soto-Fuentes V, Tenorio-Quiroz A, Gervasio-Ortiz E, Rico-Amador D, et al. Role of presurgical gut microbial diversity in Roux-en-Y gastric bypass weight-loss response: a cohort study. Lifestyle Genom. 2024;17:12–21.
Murphy R, Tsai P, Jüllig M, Liu A, Plank L, Booth M. Differential changes in gut microbiota after gastric bypass and sleeve gastrectomy bariatric surgery vary according to diabetes remission. Obes Surg. 2017;27:917–25.
Gutiérrez-Repiso C, Moreno-Indias I, Tinahones FJ. Shifts in gut microbiota and their metabolites induced by bariatric surgery. Impact of factors shaping gut microbiota on bariatric surgery outcomes. Rev Endocr Metab Disord. 2021;22:1137–56.
David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505:559–63.
Tabasi M, Eybpoosh S, Siadat SD, Elyasinia F, Soroush A, Bouzari S. Modulation of the gut microbiota and serum biomarkers after laparoscopic sleeve gastrectomy: a 1-year follow-up study. Obes Surg. 2021;31:1949–56.
Xu Z, Jiang W, Huang W, Lin Y, Chan FKL, Ng SC. Gut microbiota in patients with obesity and metabolic disorders—a systematic review. Genes Nutr. 2022;17:2.
Kayser BD, Prifti E, Lhomme M, Belda E, Dao MC, Aron-Wisnewsky J, et al. Elevated serum ceramides are linked with obesity-associated gut dysbiosis and impaired glucose metabolism. Metabolomics. 2019;15:140.
Samuel BS, Hansen EE, Manchester JK, Coutinho PM, Henrissat B, Fulton R, et al. Genomic and metabolic adaptations of Methanobrevibacter smithii to the human gut. Proc Natl Acad Sci USA. 2007;104:10643–8.
Le Chatelier E, Nielsen T, Qin J, Prifti E, Hildebrand F, Falony G, et al. Richness of human gut microbiome correlates with metabolic markers. Nature. 2013;500:541–6.
Kim MH, Yun KE, Kim J, Park E, Chang Y, Ryu S, et al. Gut microbiota and metabolic health among overweight and obese individuals. Sci Rep. 2020;10:19417.
Salehi, M, Peterson, R, Tripathy, D, Pezzica, S, DeFronzo, R, Gastaldelli, A. Insulinotropic effect of endogenous incretins is greater after gastric bypass than sleeve gastrectomy despite diminished beta-cell sensitivity to plasma incretins. Preprint. medRxiv. 2023. https://doi.org/10.1101/2023.03.28.23287755.
Acknowledgements
The authors thank Sr. Joan Badia for statistical analysis, the Bioinformatics and Biostatistics Unit at the IISPV, and the microbiome data analysis.
Funding
Research conducted in the authors’ laboratory is supported by grants from the ISCIII (PI14/00228, PI17/01503, PI20/00338 and PI23/01133 to JV, PI14/00633, PI17/00915, PI20/0114 and PI23/01289 to SP and PI14/01997; PI17/01556 and PI22/01773 to NV) and the Spanish Ministry of Science and Innovation MCIN/AEI/10.13039/501100011033 (RTI2018-093919-B-100 and PID2021-122480OB-584-100 to SFV), all co-financed by the European Consorcio Centro de Investigación Biomédica en Red (CB07708/0012), ISCIII, Ministerio de Ciencia e Innovación, and by the “La Caixa” Foundation (ID 100010434) under grant agreement LCF/PR/HR20/52400013 (to SFV). SFV and JV also acknowledge support from the Agency for Management of University Research Grants of the Generalitat de Catalunya (2021 SGR 01409, 2021 SGR 0089).
Author information
Authors and Affiliations
Contributions
RP, M-MR-P, JV, and SP contributed to the conception of the work and wrote the manuscript. LH-M and GLL contributed to the data analysis. BA performed the bioinformatics and statistical analyses. GLL, JB, JT, and AC participated in the study design. Gll, CJ, MP-D, NV, and SFV critically revised the manuscript. All authors contributed to the article and approved the submitted version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
All participants provided informed consent, and the study was approved by the ethics committee of Germans Trias I Pujol Hospital (PI-14 103). All methods were performed in accordance with the relevant guidelines and regulations.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Puig, R., Rodríguez-Peña, MM., Hernández-Montoliu, L. et al. Insulin resistance modulates gut microbiota and incretin response remodeling after bariatric surgery in severe obesity. Int J Obes (2026). https://doi.org/10.1038/s41366-025-01971-7
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41366-025-01971-7