Abstract
Background
Despite being a defining feature of metabolic syndrome (MetS), clinical assessment of IR remains challenging, due to the costs of reference methods and the numerosity of IR indices. Furthermore, to which extent IR contributes to MetS, while controlling for altered body composition, is still largely unexplored.
Objectives
The present work aims at proposing new cut points for IR among people with overweight and obesity, assessing the concordance of different IR definitions and investigating how IR interacts with body composition in predicting MetS status.
Subjects
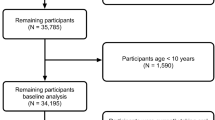
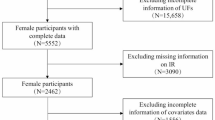
665 patients were assessed for potential enrolment, using a cross-sectional design. The following inclusion criteria were applied: age ≥18 years, body mass index ≥25 kg/m2, White European, no fulfilled criterion for diabetes mellitus, no current pregnancy.
Methods
Concordance of previously validated IR definitions was assessed by Cohen’s κ. ROC curve analysis with 5-fold cross-validation was used to determine novel cut points for IR indices based on MetS presence. Finally, mediation analysis was employed to test whether IR mediates the relationship between body composition indices (i.e., fat mass-to-fat-free mass ratio, FM:FFM and appendicular lean soft tissue-to-visceral fat area ratio, ALST:VFA) and MetS.
Results
A total of 515 patients were included in the final analysis (females: 80.9%; MetS prevalence: 40%). Overall, IR definitions which previously validated against the hyperinsulinemic-euglycemic clamp displayed the highest level of agreement. The following cut-points were identified from ROC curve analysis: ISI-Matsuda<3.33 (AUROC = 0.675, p < 0.001), HOMA-IR > 2.93 (AUROC = 0.663 p < 0.001), HOMA2-IR > 1.67 (AUROC = 0.651 p < 0.001). Finally, ALST:VFA but not FM:FFM significantly predicted MetS status independent of age, with the mediating role of ISI-Matsuda, HOMA-IR and HOMA2-IR.
Conclusions
IR indices mediated the effect of altered body composition (i.e., reduced appendicular muscularity and increased visceral adiposity) on MetS. Newly proposed diagnostic thresholds can aid in the identification of IR among patients at increased cardio-metabolic risk.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Data supporting the findings of the present study are available upon reasonable request from the corresponding author.
References
Di Cesare M, McGhie DV, Perel P, Mwangi J, Taylor S, Pervan B, et al. The Heart of the World. Glob Heart. 2024;19. https://doi.org/10.5334/GH.1288.
Ong KL, Stafford LK, McLaughlin SA, Boyko EJ, Vollset SE, Smith AE, et al. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: a systematic analysis for the Global Burden of Disease Study 2021. Lancet. 2023;402:203–34.
Mottillo S, Filion KB, Genest J, Joseph L, Pilote L, Poirier P, et al. The metabolic syndrome and cardiovascular risk: a systematic review and meta-analysis. J Am Coll Cardiol. 2010;56:1113–32.
Schulze MB, Stefan N. Metabolically healthy obesity: from epidemiology and mechanisms to clinical implications. Nat Rev Endocrinol 2024;20:633–46.
Reaven G. Syndrome X. Curr Treat Options Cardiovasc Med. 2001;3:323–32.
Reaven GM. Why Syndrome X? From Harold Himsworth to the Insulin Resistance Syndrome. Cell Metab. 2005;1:9–14.
Yudkin JS. Insulin resistance and the metabolic syndrome - or the pitfalls of epidemiology. Diabetologia. 2007;50:1576–86.
Neeland IJ, Lim S, Tchernof A, Gastaldelli A, Rangaswami J, Ndumele CE, et al. Metabolic syndrome. Nat Rev Dis Prim. 2024;10:1–22.
DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. 1979;6. https://doi.org/10.1152/AJPENDO.1979.237.3.E214.
Matsuda M. Measuring and estimating insulin resistance in clinical and research settings. Nutr Metab Cardiovasc Dis. 2010;20:79–86.
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9.
Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diab Care. 2004;27:1487–95.
Abdul-Ghani MA, Matsuda M, Balas B, DeFronzo RA. Muscle and liver insulin resistance indexes derived from the oral glucose tolerance test. Diab Care. 2007;30:89–94.
Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diab Care. 1999;22:1462–70.
Magliano DJ, Zimmet P, Shaw JE. Classification of diabetes mellitus and other categories of glucose intolerance. In DeFronzo RA, Ferrannini E, Zimmet P, Alberti KGMM, editors. International Textbook of Diabetes Mellitus. Wiley-Blackwell; 2015, p. 3–16.
Szosland K, Lewiński A. In quest for method of insulin resistance assessment in everyday clinical practice—Insulin resistance indices. Diab Metab Syndrome Clin Res Rev. 2016;10:S120–5.
Gayoso-Diz P, Otero-González A, Rodriguez-Alvarez MX, Gude F, García F, de Francisco A, et al. Insulin resistance (HOMA-IR) cut-off values and the metabolic syndrome in a general adult population: Effect of gender and age: EPIRCE cross-sectional study. BMC Endocr Disord. 2013;13:1–10.
de Paula Martins W, Santana LF, Nastri CO, Ferriani FA, de Sa MFS, dos Reis RM. Agreement among insulin sensitivity indexes on the diagnosis of insulin resistance in polycystic ovary syndrome and ovulatory women. Eur J Obstet Gynecol Reprod Biol. 2007;133:203–7.
Siervo M, Prado CM, Mire E, Broyles S, Wells JC, Heymsfield S, et al. Body composition indices of a load-capacity model: gender- and BMI-specific reference curves. Public Health Nutr. 2015;18:1245–54.
Poggiogalle E, Migliaccio S, Lenzi A, Donini LM. Treatment of body composition changes in obese and overweight older adults: insight into the phenotype of sarcopenic obesity. Endocrine. 2014;47:699–716.
Xu J, Pan X, Liang H, Lin Y, Hong Y, Si Q, et al. Association between skeletal muscle mass to visceral fat area ratio and arterial stiffness in Chinese patients with type 2 diabetes mellitus. BMC Cardiovasc Disord. 2018;18:1–8.
American Diabetes Association Professional Practice Committee. 2. Diagnosis and Classification of Diabetes: Standards of Care in Diabetes—2024. Diab Care. 2024;47:S20–S42.
WHO. Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894:1–253.
Nishida C, Ko GT, Kumanyika S. Body fat distribution and noncommunicable diseases in populations: overview of the 2008 WHO Expert Consultation on Waist Circumference and Waist–Hip Ratio. Eur J Clin Nutr. 2009;64:2–5.
Poggiogalle E, Mendes I, Ong B, Prado CM, Mocciaro G, Mazidi M, et al. Sarcopenic obesity and insulin resistance: application of novel body composition models. Nutrition. 2020;75–76:110765.
Alberti KGMM, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the Metabolic Syndrome A Joint Interim Statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009. https://doi.org/10.1161/CIRCULATIONAHA.109.192644.
Kernan WN, Inzucchi SE, Viscoli CM, Brass LM, Bravata DM, Shulman GI, et al. Pioglitazone improves insulin sensitivity among nondiabetic patients with a recent transient ischemic attack or ischemic stroke. Stroke. 2003;34:1431–6.
Stern SE, Williams K, Ferrannini E, DeFronzo RA, Bogardus C, Stern MP. Identification of individuals with insulin resistance using routine clinical measurements. Diabetes. 2005;54:333–9.
Tam CS, Xie W, Johnson WD, Cefalu WT, Redman LM, Ravussin E. Defining insulin resistance from hyperinsulinemic-euglycemic clamps. Diab Care. 2012;35:1605–10.
Hedblad B, Nilsson P, Janzon L, Berglund G. Relation between insulin resistance and carotid intima-media thickness and stenosis in non-diabetic subjects. Results from a cross-sectional study in Malmö, Sweden. Diabet Med. 2000;17:299–307.
Tomé MA, Botana MA, Cadarso-Suárez C, Rego-Iraeta A, Fernández-Mariño A, Mato JA, et al. Prevalence of metabolic syndrome in Galicia (NW Spain) on four alternative definitions and association with insulin resistance. J Endocrinol Investig. 2009;32:505–11.
Hayes AF. Introduction to mediation, moderation, and conditional process analysis: a regression-based approach. 3rd edition. The Guilford Press, 2022https://books.google.com/books/about/Introduction_to_Mediation_Moderation_and.html?hl=it&id=-P-BzgEACAAJ (accessed 21 Dec2024).
Ghasemi A, Zahediasl S. Normality tests for statistical analysis: a guide for non-statisticians. Int J Endocrinol Metab. 2012;10:486–9.
Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–74.
Thölke P, Mantilla-Ramos YJ, Abdelhedi H, Maschke C, Dehgan A, Harel Y, et al. Class imbalance should not throw you off balance: Choosing the right classifiers and performance metrics for brain decoding with imbalanced data. Neuroimage. 2023;277:120253.
Gradidge PJL, Norris SA, Jaff NG, Crowther NJ. Metabolic and body composition risk factors associated with metabolic syndrome in a cohort of women with a high prevalence of cardiometabolic disease. PLoS One. 2016;11:e0162247.
Lteif AA, Han K, Mather KJ. Obesity, insulin resistance, and the metabolic syndrome. Circulation. 2005;112:32–38.
Watson PF, Petrie A. Method agreement analysis: a review of correct methodology. Theriogenology. 2010;73:1167–79.
Staten MA, Stern MP, Miller WG, Steffes MW, Campbell SE. Insulin Assay Standardization Leading to measures of insulin sensitivity and secretion for practical clinical care FOR THE INSULIN STANDARDIZATION WORKGROUP. 2010. https://doi.org/10.2337/dc09-1206.
DeFronzo RA, Tripathy D. Skeletal muscle insulin resistance is the primary defect in type 2 diabetes. Diab Care. 2009;32:S157–63.
Muniyappa R, Tella SH, Sortur S, Mszar R, Grewal S, Abel BS, et al. Predictive accuracy of surrogate indices for hepatic and skeletal muscle insulin sensitivity. J Endocr Soc. 2019;3:108–18.
Noubiap JJ, Nansseu JR, Lontchi-Yimagou E, Nkeck JR, Nyaga UF, Ngouo AT, et al. Geographic distribution of metabolic syndrome and its components in the general adult population: a meta-analysis of global data from 28 million individuals. Diab Res Clin Pract. 2022;188:109924.
Donini LM, Busetto L, Bischoff SC, Cederholm T, Ballesteros-Pomar MD, Batsis JA, et al. Definition and diagnostic criteria for sarcopenic obesity: ESPEN and EASO consensus statement. Clin Nutr. 2022;41:990–1000.
Hocking S, Samocha-Bonet D, Milner KL, Greenfield JR, Chisholm DJ. Adiposity and insulin resistance in humans: the role of the different tissue and cellular lipid depots. Endocr Rev. 2013;34:463–500.
Yudkin JS, Eringa E, Stehouwer CDA. Vasocrine’ signalling from perivascular fat: a mechanism linking insulin resistance to vascular disease. Lancet. 2005;365:1817–20.
Otten J, Ahrén B, Olsson T. Surrogate measures of insulin sensitivity vs the hyperinsulinaemic- euglycaemic clamp: a meta-analysis. Diabetologia. 2014;57:1781–8.
Samuel VT, Petersen KF, Shulman GI. Lipid-induced insulin resistance: unravelling the mechanism. Lancet. 2010;375:2267–77.
Gao Z, Wang Z, Zhang X, Butler AA, Zuberi A, Gawronska-Kozak B, et al. Inactivation of PKCθ leads to increased susceptibility to obesity and dietary insulin resistance in mice. Am J Physiol Endocrinol Metab. 2007;292:84–91.
Yoshida Y, Chen Z, Baudier RL, Krousel-Wood M, Anderson AH, Fonseca VA, et al. Sex differences in the progression of metabolic risk factors in diabetes development. JAMA Netw Open. 2022;5:e2222070.
Acknowledgements
We acknowledge the role of the nursing personnel represented by Cinzia Estivi, Rossana Principato, Antonella Anzuini, Francesco Impelliccieri, Anna Ruggeri, Antonella Bellissari, Lidia Pawlowska, Roberto Ferri, in the assistance to patients and collection of biological samples.
Funding
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Conceptualization: EP, LMD, MS; Methodology: FF, EP, LMD, MS; Data curation FF, CP, FR, MDM, VG, EG, MM. Formal analysis: FF, AV, EP; Visualization: FF, AV, LM, CP; Investigation and resources: LMD, S.Mariani, AL, AI, LG, CL, MC; Writing–original draft preparation: all authors. Writing—review and editing: all authors. Supervision: EP, LMD, MS, S.Migliaccio; Project administration: LMD, S.Migliaccio, AL, AMI, LG, CL.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The study was conducted according to the declarations of Helsinki; protocol was approved by the Ethical Committee of the Sapienza University of Rome, Rome, Italy. Written informed consent was obtained from all the participants.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Frigerio, F., Vitozzi, A., Piciocchi, C. et al. Capturing metabolic syndrome: new thresholds for insulin resistance and novel body composition indices. Int J Obes (2026). https://doi.org/10.1038/s41366-025-01993-1
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41366-025-01993-1