Abstract
Background
Obesity represents a critical global health challenge, yet the neurocognitive distinctions in processing different rewards among individuals with overweight/obesity (OW/OB) remain poorly characterized. This meta-analysis aims to synthesize functional MRI evidence to delineate common and distinct neural abnormalities during processing of food or monetary reward cues in individuals with OW/OB compared to normal-weight (NW) controls.
Methods
Searches were conducted in Web of Science, PubMed, and PsycInfo to identify eligible citations from inception until May 2025. The review protocol was registered in PROSPERO (No. CRD42024595608). Data analyses were carried out using the Activation Likelihood Estimation (ALE) algorithm. MRIcroGL was used to show the results with MNI coordinates.
Results
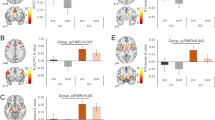
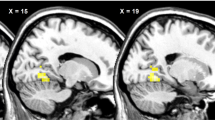
We systematically reviewed 26 studies with 1065 participants, comprising 6 monetary reward and 20 food reward studies. Overlapping reduced activation occurred in the left posterior cingulate cortex (PCC) and insula across both reward types; the left middle frontal gyrus (MFG) exhibited decreased activation in response to food reward cues, while it showed increased activation in response to monetary reward cues in individuals with OW/OB. During food-reward tasks, individuals with OW/OB exhibited increased activation in the bilateral caudate nucleus, hippocampus, anterior cingulate cortex (ACC), and medial prefrontal cortex, alongside decreased activation in amygdala responses. For monetary-reward tasks, increased activation was observed in the right lateral nucleus and hypothalamus, while decreased activation was observed in the right subthalamic nucleus (STN) and posterior ventral lateral nucleus.
Conclusion
Obesity represents a critical global health challenge, yet the neurocognitive distinctions in processing different rewards among individuals with OW/OB remain poorly characterized. Our findings reveal both dissociable and overlapping neural alterations during the processing of primary (food) versus secondary (monetary) rewards in OW/OB, implicating altered reward sensitivity, decision-making, and inhibitory control. The results underscore the necessity for reward-type-specific interventions targeting these neural mechanisms to address obesity-related dysregulation.

Contrasts in brain activation during food and monetary reward processing between individuals with overweight/obesity (OW/OB) and normal weight (NW).
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Phelps NH, Singleton RK, Zhou B, Heap RA, Mishra A, Bennett JE, et al. Worldwide trends in underweight and obesity from 1990 to 2022: a pooled analysis of 3663 population-representative studies with 222 million children, adolescents, and adults. Lancet. 2024;403:1027–50.
World Obesity Federation. World Obesity Atlas 2025. 2025. Available from: https://www.worldobesity.org/resources/resource-library/world-obesity-atlas-2025 (accessed 10 May 2025).
Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:766–81.
Grillo CA, Mulder P, Macht VA, Kaigler KF, Wilson SP, Wilson MA, et al. Dietary restriction reverses obesity-induced anhedonia. Physiol Behav. 2014;128:126–32.
Lin X, Li H. Obesity: epidemiology, pathophysiology, and therapeutics. Front Endocrinol (Lausanne). 2021;12:706978.
Davis C, Patte K, Levitan R, Reid C, Tweed S, Curtis C. From motivation to behaviour: a model of reward sensitivity, overeating, and food preferences in the risk profile for obesity. Appetite. 2007;48:12–19.
Mansur RB, Subramaniapillai M, Zuckerman H, Park C, Iacobucci M, Lee Y, et al. Effort-based decision-making is affected by overweight/obesity in major depressive disorder. J Affect Disord. 2019;256:221–7.
Nusslock R, Alloy LB. Reward processing and mood-related symptoms: An RDoC and translational neuroscience perspective. J Affect Disord. 2017;216:3–16.
Meng X, Huang D, Ao H, Wang X, Gao X. Food cue recruits increased reward processing and decreased inhibitory control processing in the obese/overweight: an activation C likelihood estimation meta-analysis of fMRI studies. Obes Res Clin Pract. 2020;14:127–35.
Diekhof EK, Kaps L, Falkai P, Gruber O. The role of the human ventral striatum and the medial orbitofrontal cortex in the representation of reward magnitude—an activation likelihood estimation meta-analysis of neuroimaging studies of passive reward expectancy and outcome processing. Neuropsychologia. 2012;50:1252–66.
Sescousse G, Caldú X, Segura B, Dreher JC. Processing of primary and secondary rewards: a quantitative meta-analysis and review of human functional neuroimaging studies. Neurosci Biobehav Rev. 2013;37:681–96.
Verdejo-Roman J, Vilar-Lopez R, Navas JF, Soriano-Mas C, Verdejo-Garcia A. Brain reward system’s alterations in response to food and monetary stimuli in overweight and obese individuals. Hum Brain Mapp. 2017;38:666–77.
Gill H, Gill B, Lipsitz O, Rodrigues NB, Cha DS, El-Halabi S, et al. The impact of overweight/obesity on monetary reward processing: a systematic review. J Psychiatr Res. 2021;137:456–64.
Janssen LK, Duif I, van Loon I, Wegman J, de Vries JHM, Cools R, et al. Loss of lateral prefrontal cortex control in food-directed attention and goal-directed food choice in obesity. NeuroImage. 2017;146:148–56.
Opel N, Redlich R, Grotegerd D, Dohm K, Haupenthal C, Heindel W, et al. Enhanced neural responsiveness to reward associated with obesity in the absence of food-related stimuli. Hum Brain Mapp. 2015;36:2330–7.
Mata F, Treadway M, Kwok A, Truby H, Yucel M, Stout JC, et al. Reduced willingness to expend effort for reward in obesity: link to adherence to a 3-month weight loss intervention. Obesity. 2017;25:1676–81.
Kubanek J, Snyder LH. Reward-based decision signals in parietal cortex are partially embodied. J Neurosci. 2015;35:4869–81.
Bogdanov VB, Bogdanova OV, Dexpert S, Delgado I, Beyer H, Aubert A, et al. Reward-related brain activity and behavior are associated with peripheral ghrelin levels in obesity. Psychoneuroendocrinology. 2020;112:104520.
Kube J, Mathar D, Horstmann A, Kotz SA, Villringer A, Neumann J. Altered monetary loss processing and reinforcement-based learning in individuals with obesity. Brain Imaging Behav. 2018;12:1431–49.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev. 2021;10:89.
Eickhoff SB, Laird AR, Grefkes C, Wang LE, Zilles K, Fox PT. Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: a random-effects approach based on empirical estimates of spatial uncertainty. Hum Brain Mapp. 2009;30:2907–26.
Laird AR, Fox PM, Price CJ, Glahn DC, Uecker AM, Lancaster JL, et al. ALE meta-analysis: controlling the false discovery rate and performing statistical contrasts. Hum Brain Mapp. 2005;25:155–64.
Turkeltaub PE, Eden GF, Jones KM, Zeffiro TA. Meta-analysis of the functional neuroanatomy of single-word reading: method and validation. Neuroimage. 2002;16:765–80.
Dahlberg LS, Becerra L, Borsook D, Linnman C. Brain changes after spinal cord injury, a quantitative meta-analysis and review. Neurosci Biobehav Rev. 2018;90:272–93.
McCoy AN, Crowley JC, Haghighian G, Dean HL, Platt ML. Saccade reward signals in posterior cingulate cortex. Neuron. 2003;40:1031–40.
Pearson JM, Heilbronner SR, Barack DL, Hayden BY, Platt ML. Posterior cingulate cortex: adapting behavior to a changing world. Trends Cogn Sci. 2011;15:143–51.
Bragulat V, Dzemidzic M, Bruno C, Cox CA, Talavage T, Considine RV, et al. Food-related odor probes of brain reward circuits during hunger: a pilot fMRI study. Obesity. 2010;18:1566–71.
Balodis IM, Kober H, Worhunsky PD, White MA, Stevens MC, Pearlson GD, et al. Monetary reward processing in obese individuals with and without binge eating disorder. Biol Psychiatry. 2013;73:877–86.
Rothemund Y, Preuschhof C, Bohner G, Bauknecht H-C, Klingebiel R, Flor H, et al. Differential activation of the dorsal striatum by high-calorie visual food stimuli in obese individuals. NeuroImage. 2007;37:410–21.
DelParigi A, Chen K, Salbe AD, Hill JO, Wing RR, Reiman EM, et al. Persistence of abnormal neural responses to a meal in postobese individuals. Int J Obes. 2004;28:370–7.
Simon JJ, Skunde M, Walther S, Bendszus M, Herzog W, Friederich H-C. Neural signature of food reward processing in bulimic-type eating disorders. Soc Cogn Affect Neurosci. 2016;11:1393–401.
Fuster JM. 96Physiology of Executive functions: the perception-action cycle. In: Stuss DT, Knight RT (eds). Principles of Frontal Lobe Function. Oxford University Press, 2002.
García-García I, Jurado MÁ, Garolera M, Marqués-Iturria I, Horstmann A, Segura B, et al. Functional network centrality in obesity: a resting-state and task fMRI study. Psychiatry Res Neuroimaging. 2015;233:331–8.
Liu Y, Chen H. The neural mechanisms and interventions of working memory in overweight/obese individuals. J Progress Psychol. 2023;31:1775–84.
Lopez RB, Chen P-HA, Huckins JF, Hofmann W, Kelley WM, Heatherton TF. A balance of activity in brain control and reward systems predicts self-regulatory outcomes. Soc Cogn Affect Neurosci. 2017;12:832–8.
Martín-Luengo B, Vorobiova AN, Feurra M, Myachykov A, Shtyrov Y. Transcranial magnetic stimulation of the left middle frontal gyrus modulates the information people communicate in different social contexts. Sci Rep. 2023;13:9995.
Cristofori I, Harquel S, Isnard J, Mauguière F, Sirigu A. Monetary reward suppresses anterior insula activity during social pain. Soc Cogn Affect Neurosci. 2015;10:1668–76.
Volkow ND, Wang GJ, Baler RD. Reward, dopamine and the control of food intake: implications for obesity. Trends Cogn Sci. 2011;15:37–46.
Nummenmaa L, Hirvonen J, Hannukainen JC, Immonen H, Lindroos MM, Salminen P, et al. Dorsal striatum and its limbic connectivity mediate abnormal anticipatory reward processing in obesity. Plos One. 2012;7:e31089.
Stoeckel LE, Weller RE, Cook EW, Twieg III, Knowlton DB, Cox RC. JE. Widespread reward-system activation in obese women in response to pictures of high-calorie foods. Neuroimage. 2008;41:636–47.
Stice E, Spoor S, Bohon C, Veldhuizen MG, Small DM. Relation of reward from food intake and anticipated food intake to obesity: a functional magnetic resonance imaging study. J Abnorm Psychol. 2008;117:924–35.
Miranda-Olivos R, Steward T, Martinez-Zalacain I, Mestre-Bach G, Juaneda-Segui A, Jimenez-Murcia S, et al. The neural correlates of delay discounting in obesity and binge eating disorder. J Behav Addict. 2021;10:498–507.
Steward T, Menchon JM, Jiménez-Murcia S, Soriano-Mas C, Fernandez-Aranda F. Neural network alterations across eating disorders: a narrative review of fMRI studies. 2017;16:1150–63.
Pursey KM, Stanwell P, Callister RJ, Brain K, Collins CE, Burrows TL. Neural responses to visual food cues according to weight status: a systematic review of functional magnetic resonance imaging studies. Front Nutr. 2014;1:7.
Berridge KC. Food reward: brain substrates of wanting and liking. Neurosci Biobehav Rev. 1996;20:1–25.
Kelley AE. Ventral striatal control of appetitive motivation: role in ingestive behavior and reward-related learning. Neurosci Biobehav Rev. 2004;27:765–76.
Yuan Z, Qi Z, Wang R, Cui Y, An S, Wu G, et al. A corticoamygdalar pathway controls reward devaluation and depression using dynamic inhibition code. Neuron. 2023;111:3837–.e5.
Allsop SA, Wichmann R, Mills F, Burgos-Robles A, Chang C-J, Felix-Ortiz AC, et al. Corticoamygdala Transfer of Socially Derived Information Gates Observational Learning. Cell. 2018;173:1329–.e18.
Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4:215–22.
Cohen Y, Schneidman E, Paz R. The geometry of neuronal representations during rule learning reveals complementary roles of cingulate cortex and putamen. Neuron. 2021;109:839–.e9.
Horstmann A, Dietrich A, Mathar D, Pössel M, Villringer A, Neumann J. Slave to habit? Obesity is associated with decreased behavioural sensitivity to reward devaluation. Appetite. 2015;87:175–83.
Monosov IE. Anterior cingulate is a source of valence-specific information about value and uncertainty. Nat Commun. 2017;8:134.
Sallet J, Quilodran R, Rothé M, Vezoli J, Joseph J-P, Procyk E. Expectations, gains, and losses in the anterior cingulate cortex. Cogn Affect Behav Neurosci. 2007;7:327–36.
Wee RWS, Mishchanchuk K, Alsubaie R, Church TW, Gold MG, Macaskill AF. Internal-state-dependent control of feeding behavior via hippocampal ghrelin signaling. Neuron. 2024;112:288-305.e7.
Paradiso S. The emotional brain: the mysterious underpinnings of emotional life. Am J Psychiatry. 1998;155:570–570.
Pauli WM, Hazy TE, O’Reilly RC. Expectancy, ambiguity, and behavioral flexibility: separable and complementary roles of the orbital frontal cortex and amygdala in processing reward expectancies. J Cogn Neurosci. 2012;24:351–66.
Ghobadi-Azbari P, Mahdavifar Khayati R, Sangchooli A, Ekhtiari H. Task-dependent effective connectivity of the reward network during food cue-reactivity: a dynamic causal modeling investigation. Front Behav Neurosci. 2022;16:899605.
Ottenheimer DJ, Bari BA, Sutlief E, Fraser KM, Kim TH, Richard JM, et al. A quantitative reward prediction error signal in the ventral pallidum. Nat Neurosci. 2020;23:1267 +.
Vachez YM, Tooley JR, Abiraman K, Matikainen-Ankney B, Casey E, Earnest T, et al. Ventral arkypallidal neurons inhibit accumbal firing to promote reward consumption. Nat Neurosci. 2021;24:379–90.
Serranová T, Jech R, Dušek P, Sieger T, Růžička F, Urgošík D, et al. Subthalamic nucleus stimulation affects incentive salience attribution in Parkinson’s disease. 2011;26:2260–6.
Aiello M, Terenzi D, Furlanis G, Catalan M, Manganotti P, Eleopra R, et al. Deep brain stimulation of the subthalamic nucleus and the temporal discounting of primary and secondary rewards. J Neurol. 2019;266:1113–9.
Soh C, Hervault M, Chalkley NH, Moore CM, Rohl A, Zhang Q, et al. The human subthalamic nucleus transiently inhibits active attentional processes. Brain J Neurol. 2024;147:3204–15.
Mechelmans DJ, Strelchuk D, Donamayor N, Banca P, Robbins TW, Baek K, et al. Reward sensitivity and waiting impulsivity: shift towards reward valuation away from action control. Int J Neuropsychopharmacol. 2017;20:971–8.
Concetti C, Peleg-Raibstein D, Burdakov D. Hypothalamic MCH neurons: from feeding to cognitive control. Funct (Oxf Engl). 2024;5:zqad059.
Ma T, Wong SZH, Lee B, Ming G-l, Song H. Decoding neuronal composition and ontogeny of individual hypothalamic nuclei. Neuron. 2021;109:1150–.e6.
Ogawa A, Osada T, Tanaka M, Suda A, Nakajima K, Oka S, et al. Hypothalamic interaction with reward-related regions during subjective evaluation of foods. NeuroImage. 2022;264:119744.
Groos D, Reuss AM, Rupprecht P, Stachniak T, Lewis C, Han S, et al. A distinct hypothalamus–habenula circuit governs risk preference. Nature Neurosci. 2025; 28:361–373.
Hare TA, O’Doherty J, Camerer CF, Schultz W, Rangel A. Dissociating the role of the orbitofrontal cortex and the striatum in the computation of goal values and prediction errors. J Neurosci. 2008;28:5623–30.
Devoto F, Ferrulli A, Banfi G, Luzi L, Zapparoli L, Paulesu E. How images of food become cravingly salient in obesity. Obesity. 2023;31:2294–2303.
Kishinevsky FI, Cox JE, Murdaugh DL, Stoeckel LE, Cook EW, Weller RE. fMRI reactivity on a delay discounting task predicts weight gain in obese women. Appetite. 2012;58:582–92.
Batterink L, Yokum S, Stice E. Body mass correlates inversely with inhibitory control in response to food among adolescent girls: An fMRI study. NeuroImage. 2010;52:1696–703.
Boutelle KN, Wierenga CE, Bischoff-Grethe A, Melrose AJ, Grenesko-Stevens E, Paulus MP, et al. Increased brain response to appetitive tastes in the insula and amygdala in obese compared with healthy weight children when sated. Int J Obes. 2015;39:620–8.
Funding
This work was funded by the National Natural Science Foundation of China (32300926) and the Provincial Key Research Project of Henan Province (No. 232102310081). The funding agents had no role in the design and conduct of the study, including the collection, management, analysis, and interpretation of the data and the preparation, review, or approval of the manuscript.
Author information
Authors and Affiliations
Contributions
Na Wang and Mingyue Xiao proposed the research question, designed the study protocol, wrote the initial draft of the paper, and interpreted the analysis results; Na Wang and Ruyin Yuan were responsible for data collection and screening and participated in data analysis; Chenxuan Lu and Lam ChiFong assisted with data collection and participated in discussions of the paper; and Bing Cao, Mingyue Xiao, and Hong Chen provided methodological guidance and reviewed and revised the paper, ensuring the scientific rigor and accuracy of the research. All of the authors contributed substantially to the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, N., Xiao, M., Hou, X. et al. Differentiating the abnormalities of food and monetary reward cue processing associated with overweight/obesity: an ALE meta-analysis. Int J Obes (2026). https://doi.org/10.1038/s41366-026-02026-1
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41366-026-02026-1