Abstract
Background
Prenatal air pollution (AP) exposure has been linked to postpartum psychological functioning, impacting health outcomes in both women and children. Extant studies primarily focused on individual pollutants.
Objective
To assess the association between prenatal exposure to a mixture of seven AP components and postpartum psychological functioning using daily exposure data and data-driven statistical methods.
Methods
Analyses included 981 women recruited at 24.0 ± 9.9 weeks gestation and followed longitudinally. We estimated prenatal daily exposure levels for constituents of fine particles [elemental carbon (EC), organic carbon (OC), nitrate (NO3−), sulfate (SO42−), ammonium (NH4+)], nitrogen dioxide (NO2), and ozone (O3) using validated global 3-D chemical-transport models and satellite-based hybrid models based on residential addresses. Edinburgh Postnatal Depression Scale (EPDS) was administered to participants to derive a total EPDS score and the subconstruct scores for anhedonia and depressive symptoms. A distributed lag model (DLM) was employed within Bayesian Kernel Machine Regression (BKMR) to develop time-weighted exposure profile for each pollutant. These exposures were then input into a Weighted Quantile Sum (WQS) regression to estimate an overall mixture effect, adjusted for maternal age, education, race/ethnicity, season of delivery, and delivery year. Effect modification by race/ethnicity and fetal sex was also examined.
Results
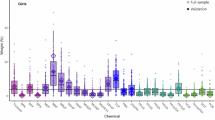
Women were primarily Hispanic (51%) and Black (32%) reporting ≤12 years of education (58%). Prenatal exposure to an AP mixture was significantly associated with increased anhedonia subscale z-scores, particularly in Hispanics (β = 0.07, 95%CI = 0.004–0.13, per unit increase in WQS index). It was also borderline associated with increased total EPDS (β = 0.11, 95%CI = 0.00–0.22) and depressive symptom subscale (β = 0.09, 95%CI = −0.02 to 0.19) z-scores, particularly among Hispanic women who gave birth to a male infant. Sulfate (SO42−), O3 and OC were major contributors to these associations.
Impact
-
This study utilizes an advanced data-driven approach to examine the temporally- and mixture-weighted effects of prenatal air pollution exposure on postpartum psychological functioning. We found that exposure to a prenatal air pollution mixture predicted poorer postpartum psychological functioning, particularly anhedonia symptoms in Hispanic women. Findings underscore the importance of considering both exposure mixtures as well as potential modifying factors to better help identify particular pollutants that drive effects and susceptible populations, which can inform more effective intervention strategies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 print issues and online access
$259.00 per year
only $43.17 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
The data presented in this study are available from the author on reasonable request. The data are not publicly available due to their containing confidential and protected health information that could compromise the privacy of research participants.
References
Hahn-Holbrook J, Cornwell-Hinrichs T, Anaya I. Economic and Health Predictors of National Postpartum Depression Prevalence: A Systematic Review, Meta-analysis, and Meta-Regression of 291 Studies from 56 Countries. Front Psychiatry. 2017;8:248.
Slomian J, Honvo G, Emonts P, Reginster JY, Bruyère O. Consequences of maternal postpartum depression: A systematic review of maternal and infant outcomes. Womens Health. 2019;15:1745506519844044.
WHO. World Health Organizatioin (WHO). WHO recommendations on maternal and newborn care for a positive postnatal experience. 2022. https://www.who.int/publications/i/item/9789240045989 [Accessed December 2, 2023].
Braithwaite I, Zhang S, Kirkbride JB, Osborn DPJ, Hayes JF. Air Pollution (Particulate Matter) Exposure and Associations with Depression, Anxiety, Bipolar, Psychosis and Suicide Risk: A Systematic Review and Meta-Analysis. Environ Health Perspect. 2019;127:126002.
Jin J, Xu Z, Beevers SD, Huang J, Kelly F, Li G. Long-term ambient ozone, omega-3 fatty acid, genetic susceptibility, and risk of mental disorders among middle-aged and older adults in UK biobank. Environ Res. 2023;243:117825.
Sun Y, Headon KS, Jiao A, Slezak JM, Avila CC, Chiu VY, et al. Association of Antepartum and Postpartum Air Pollution Exposure With Postpartum Depression in Southern California. JAMA Netw Open. 2023;6:e2338315-e.
Bastain TM, Chavez T, Habre R, Hernandez-Castro I, Grubbs B, Toledo-Corral CM, et al. Prenatal ambient air pollution and maternal depression at 12 months postpartum in the MADRES pregnancy cohort. Environ Health. 2021;20:121.
Niedzwiecki MM, Rosa MJ, Solano-González M, Kloog I, Just AC, Martínez-Medina S, et al. Particulate air pollution exposure during pregnancy and postpartum depression symptoms in women in Mexico City. Environ Int. 2020;134:105325.
Pourhoseini SA, Akbary A, Mahmoudi H, Akbari M, Heydari ST. Association between prenatal period exposure to ambient air pollutants and development of postpartum depression: a systematic review and meta-analysis. Int J Environ Health Res. 2024;34:455–65.
Sheffield PE, Speranza R, Chiu YM, Hsu HL, Curtin PC, Renzetti S, et al. Association between particulate air pollution exposure during pregnancy and postpartum maternal psychological functioning. PLoS One. 2018;13:e0195267.
Berk M, Williams LJ, Jacka FN, O’Neil A, Pasco JA, Moylan S, et al. So depression is an inflammatory disease, but where does the inflammation come from? BMC Med. 2013;11:200.
Fialova L, Malbohan I, Kalousova M, Soukupova J, Krofta L, Stipek S, et al. Oxidative stress and inflammation in pregnancy. Scand J Clin Lab Invest. 2006;66:121–7.
Grevendonk L, Janssen BG, Vanpoucke C, Lefebvre W, Hoxha M, Bollati V, et al. Mitochondrial oxidative DNA damage and exposure to particulate air pollution in mother-newborn pairs. Environ Health. 2016;15:10.
Rier SE. Environmental immune disruption: a comorbidity factor for reproduction? Fertil Steril. 2008;89:e103–8.
Posillico CK, Schwarz JM. An investigation into the effects of antenatal stressors on the postpartum neuroimmune profile and depressive-like behaviors. Behav Brain Res. 2016;298:218–28.
Capuron L, Miller AH. Immune system to brain signaling: neuropsychopharmacological implications. Pharm Ther. 2011;130:226–38.
Cooper JA, Arulpragasam AR, Treadway MT. Anhedonia in depression: biological mechanisms and computational models. Curr Opin Behav Sci. 2018;22:128–35.
Slavich GM, Irwin MR. From stress to inflammation and major depressive disorder: a social signal transduction theory of depression. Psychol Bull. 2014;140:774–815.
Cowell W, Colicino E, Askowitz T, Nentin F, Wright RJ. Fetal sex and maternal postpartum depressive symptoms: findings from two prospective pregnancy cohorts. Biol Sex Differ. 2021;12:6.
Clifton VL. Review: Sex and the human placenta: mediating differential strategies of fetal growth and survival. Placenta. 2010;31:S33–9.
Slavich GM, Sacher J. Stress, sex hormones, inflammation, and major depressive disorder: Extending Social Signal Transduction Theory of Depression to account for sex differences in mood disorders. Psychopharmacology. 2019;236:3063–79.
Ladd-Acosta C, Feinberg JI, Brown SC, Lurmann FW, Croen LA, Hertz-Picciotto I, et al. Epigenetic marks of prenatal air pollution exposure found in multiple tissues relevant for child health. Environ Int. 2019;126:363–76.
Morello-Frosch R, Zuk M, Jerrett M, Shamasunder B, Kyle AD. Understanding the cumulative impacts of inequalities in environmental health: implications for policy. Health Aff. 2011;30:879–87.
Dominici F, Peng RD, Barr CD, Bell ML. Protecting human health from air pollution: shifting from a single-pollutant to a multipollutant approach. Epidemiology. 2010;21:187–94.
Wilson A, Hsu H-HL, Chiu Y-HM, Wright RO, Wright RJ, Coull BA. Kernel machine and distributed lag models for assessing windows of susceptibility to environmental mixtures in children’s health studies. Ann Appl Stat. 2022;16:1090–110. 21
Kelly FJ, Fussell JC. Size, source and chemical composition as determinants of toxicity attributable to ambient particulate matter. Atmos Environ. 2012;60:504–26.
Bell ML, Dominici F, Ebisu K, Zeger SL, Samet JM. Spatial and temporal variation in PM(2.5) chemical composition in the United States for health effects studies. Environ Health Perspect. 2007;115:989–95.
Morris RH, Counsell SJ, McGonnell IM, Thornton C. Early life exposure to air pollution impacts neuronal and glial cell function leading to impaired neurodevelopment. Bioessays. 2021;43:e2000288.
Kortenkamp A, Faust M, Scholze M, Backhaus T. Low-level exposure to multiple chemicals: reason for human health concerns? Environ Health Perspect. 2007;115:106–14.
Braun JM, Gennings C, Hauser R, Webster TF. What Can Epidemiological Studies Tell Us about the Impact of Chemical Mixtures on Human Health? Environ Health Perspect. 2016;124:A6–9.
Myers S, Johns SE. Male infants and birth complications are associated with increased incidence of postnatal depression. Soc Sci Med. 2019;220:56–64.
Di Q, Koutrakis P, Schwartz J. A hybrid prediction model for PM2.5 mass and components using a chemical transport model and land use regression. Atmos Environ. 2016;131:390–9.
Di Q, Amini H, Shi L, Kloog I, Silvern R, Kelly J, et al. Assessing NO(2) Concentration and Model Uncertainty with High Spatiotemporal Resolution across the Contiguous United States Using Ensemble Model Averaging. Environ Sci Technol. 2020;54:1372–84.
Di Q, Rowland S, Koutrakis P, Schwartz J. A hybrid model for spatially and temporally resolved ozone exposures in the continental United States. J Air Waste Manag Assoc. 2017;67:39–52.
Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150:782–6.
Coates R, Ayers S, de Visser R. Factor structure of the Edinburgh Postnatal Depression Scale in a population-based sample. Psychol Assess. 2017;29:1016–27.
Cunningham NK, Brown PM, Page AC. Does the Edinburgh Postnatal Depression Scale measure the same constructs across time? Arch Womens Ment Health. 2015;18:793–804.
Chiu YM, Sheffield PE, Hsu HL, Goldstein J, Curtin PC, Wright RJ. Subconstructs of the Edinburgh Postnatal Depression Scale in a multi-ethnic inner-city population in the U.S. Arch Women’s Ment Health. 2017;20:803–10.
The PACT Consortium. Postpartum Depression: Action Towards Causes Treatment (PACT) Consortium. Heterogeneity of postpartum depression: a latent class analysis. Lancet Psychiatry. 2015;2:59–67.
Bobb JF, Valeri L, Claus Henn B, Christiani DC, Wright RO, Mazumdar M, et al. Bayesian kernel machine regression for estimating the health effects of multi-pollutant mixtures. Biostatistics. 2015;16:493–508.
Carrico C, Gennings C, Wheeler DC, Factor-Litvak P. Characterization of Weighted Quantile Sum Regression for Highly Correlated Data in a Risk Analysis Setting. J Agric Biol Environ Stat. 2015;20:100–20.
Gennings C, Sabo R, Carney E. Identifying subsets of complex mixtures most associated with complex diseases: polychlorinated biphenyls and endometriosis as a case study. Epidemiology. 2010;21:S77–84.
Tanner EM, Bornehag CG, Gennings C. Repeated holdout validation for weighted quantile sum regression. MethodsX. 2019;6:2855–60.
Duan CC, Li C, Xu JJ, He YC, Xu HL, Zhang D, et al. Association between prenatal exposure to ambient air pollutants and postpartum depressive symptoms: A multi-city cohort study. Environ Res. 2022;209:112786.
Shih P, Wu C-D, Chiang T-l, Chen P-C, Su T-C, Cheng T-J, et al. The association between postpartum depression and air pollution during pregnancy and postpartum period: a national population study in Taiwan. Environ Res Lett. 2021;16:084021.
Masselot P, Sera F, Schneider R, Kan H, Lavigne E, Stafoggia M, et al. Differential Mortality Risks Associated With PM2.5 Components: A Multi-Country, Multi-City Study. Epidemiology. 2022;33:167–75.
Borroni E, Pesatori AC, Bollati V, Buoli M, Carugno M. Air pollution exposure and depression: A comprehensive updated systematic review and meta-analysis. Environ Pollut. 2022;292:118245.
Szyszkowicz M, Kousha T, Kingsbury M, Colman I. Air Pollution and Emergency Department Visits for Depression: A Multicity Case-Crossover Study. Environ Health Insights. 2016;10:155–61.
Yuan Y, Wang K, Wang Z, Zheng H, Ma Z, Liu R, et al. Ambient ozone exposure and depression among middle-aged and older adults: Nationwide longitudinal evidence in China. Int J Hyg Environ Health. 2023;251:114185.
Manczak EM, Miller JG, Gotlib IH. Census tract ambient ozone predicts trajectories of depressive symptoms in adolescents. Dev Psychol. 2022;58:485–92.
Markevych I, Schoierer J, Hartig T, Chudnovsky A, Hystad P, Dzhambov AM, et al. Exploring pathways linking greenspace to health: Theoretical and methodological guidance. Environ Res. 2017;158:301–17.
Li H, Zhang S, Qian ZM, Xie XH, Luo Y, Han R, et al. Short-term effects of air pollution on cause-specific mental disorders in three subtropical Chinese cities. Environ Res. 2020;191:110214.
Wei F, Wu M, Qian S, Li D, Jin M, Wang J, et al. Association between short-term exposure to ambient air pollution and hospital visits for depression in China. Sci Total Environ. 2020;724:138207.
Kim YK, Na KS, Myint AM, Leonard BE. The role of pro-inflammatory cytokines in neuroinflammation, neurogenesis and the neuroendocrine system in major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2016;64:277–84.
Salari M, Zare Mehrjerdi F, Yadegari M, Rezvani ME, Shahrokhi Raeini A. Antidepressant-like effect of endogenous SO(2) on depression caused by chronic unpredictable mild stress. Naunyn Schmiedebergs Arch Pharm. 2023;396:1325–36.
Zhou YM, An SJ, Tang EJ, Xu C, Cao Y, Liu XL, et al. Association between short-term ambient air pollution exposure and depression outpatient visits in cold seasons: a time-series analysis in northwestern China. J Toxicol Environ Health A. 2021;84:389–98.
Wilson A, Chiu YM, Hsu HL, Wright RO, Wright RJ, Coull BA. Potential for Bias When Estimating Critical Windows for Air Pollution in Children’s Health. Am J Epidemiol. 2017;186:1281–9.
Payne JL, Maguire J. Pathophysiological mechanisms implicated in postpartum depression. Front Neuroendocrinol. 2019;52:165–80.
MohanKumar SMJ, Campbell A, Block M, Veronesi B. Particulate matter, oxidative stress and neurotoxicity. NeuroToxicology. 2008;29:479–88.
Yan Z, Liu Y-m, Wu W-d, Jiang Y, Zhuo L-B. Combined exposure of heat stress and ozone enhanced cognitive impairment via neuroinflammation and blood brain barrier disruption in male rats. Sci Total Environ. 2023;857:159599.
Block ML, Elder A, Auten RL, Bilbo SD, Chen H, Chen JC, et al. The outdoor air pollution and brain health workshop. Neurotoxicology. 2012;33:972–84.
Miller AH, Raison CL. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol. 2016;16:22–34.
Ahlers NE, Weiss SJ. Exposure to particulate matter, prenatal depressive symptoms and HPA axis dysregulation. Heliyon. 2021;7:e07166.
Jia Z, Wei Y, Li X, Yang L, Liu H, Guo C, et al. Exposure to Ambient Air Particles Increases the Risk of Mental Disorder: Findings from a Natural Experiment in Beijing. Int J Environ Res Public Health. 2018;15:160.
Rivas-Arancibia S, Guevara-Guzmán R, López-Vidal Y, Rodríguez-Martínez E, Zanardo-Gomes M, Angoa-Pérez M, et al. Oxidative Stress Caused by Ozone Exposure Induces Loss of Brain Repair in the Hippocampus of Adult Rats. Toxicological Sci. 2009;113:187–97.
Pan Z, Rosenblat JD, Swardfager W, McIntyre RS. Role of Proinflammatory Cytokines in Dopaminergic System Disturbances, Implications for Anhedonic Features of MDD. Curr Pharm Des. 2017;23:2065–72.
Bramble K, Blanco MN, Doubleday A, Gassett AJ, Hajat A, Marshall JD, et al. Exposure Disparities by Income, Race and Ethnicity, and Historic Redlining Grade in the Greater Seattle Area for Ultrafine Particles and Other Air Pollutants. Environ Health Perspect. 2023;131:77004.
Liu J, Clark LP, Bechle MJ, Hajat A, Kim SY, Robinson AL, et al. Disparities in Air Pollution Exposure in the United States by Race/Ethnicity and Income, 1990-2010. Environ Health Perspect. 2021;129:127005.
Bell ML, Ebisu K, Belanger K. Ambient air pollution and low birth weight in Connecticut and Massachusetts. Environ Health Perspect. 2007;115:1118–24.
Chiu YHM, Carroll KN, Coull BA, Kannan S, Wilson A, Wright RJ. Prenatal Fine Particulate Matter, Maternal Micronutrient Antioxidant Intake, and Early Childhood Repeated Wheeze: Effect Modification by Race/Ethnicity and Sex. Antioxidants (Basel). 2022;11:366.
Hajat A, Diez-Roux AV, Adar SD, Auchincloss AH, Lovasi GS, O’Neill MS, et al. Air pollution and individual and neighborhood socioeconomic status: evidence from the Multi-Ethnic Study of Atherosclerosis (MESA). Environ Health Perspect. 2013;121:1325–33.
Chakraborty J, Aun JJ. Social Inequities in Exposure to Traffic-Related Air and Noise Pollution at Public Schools in Texas. Int J Environ Res Public Health. 2023;20:5308.
Liu CH, Tronick E. Prevalence and predictors of maternal postpartum depressed mood and anhedonia by race and ethnicity. Epidemiol Psychiatr Sci. 2014;23:201–9.
Gee GC, Payne-Sturges DC. Environmental health disparities: a framework integrating psychosocial and environmental concepts. Environ Health Perspect. 2004;112:1645–53.
Rosenfeld CS. Sex-Specific Placental Responses in Fetal Development. Endocrinology. 2015;156:3422–34.
Garcia-Esteve L, Ascaso C, Ojuel J, Navarro P. Validation of the Edinburgh Postnatal Depression Scale (EPDS) in Spanish mothers. J Affect Disord. 2003;75:71–6.
Acknowledgements
The Asthma Coalition on Community, Environment, and Social Stress (ACCESS) and PRogramming of Intergenerational Stress Mechanisms (PRISM) projects have been funded by National Institutes of Health (NIH) grants R01 HL095606, R01 ES010932, U01 HL072494, R01 HL080674, R01 MD006086, and UG3 OD023337; phenotyping and/or statistical support was funded by P30 ES000002, P30 ES023515, and UL1 TR004419.
Author information
Authors and Affiliations
Contributions
Chiu YHM: Conceptualization, Methodology, Formal Analysis, Data Curation, Visualization, Writing - Original Draft. Coull BA: Methodology, Software, Writing - Review & Editing. Wilson A: Methodology, Software, Writing - Review & Editing. Hsu HHL: Methodology, Formal Analysis, Visualization, Writing - Original Draft. Xhani N: Data Curation. Nentin F: Investigation. Deli BC: Investigation. Schwartz J: Resources, Writing - Review & Editing. Colicino E: Methodology, Software, Writing - Review & Editing. Wright RO: Methodology, Writing - Review & Editing. Wright RJ: Supervision, Funding Acquisition, Conceptualization, Methodology, Resources, Project Administration, Writing - Original Draft.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
All study protocols were reviewed and approved by the Program for the Protection of Human Subjects at the Icahn School of Medicine at Mount Sinai for the ACCESS project (IRB#12-00661) and the PRISM project (IRB#12-00875). Informed written consent was obtained from all participants. The authors confirm that all procedures contributing to this work comply with the ethical standards as outlined in the Declaration of Helsinki.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chiu, YH.M., Coull, B.A., Wilson, A. et al. Air pollution mixture exposure during pregnancy and postpartum psychological functioning: racial/ethnic- and fetal sex-specific associations. J Expo Sci Environ Epidemiol 35, 548–556 (2025). https://doi.org/10.1038/s41370-024-00726-2
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41370-024-00726-2