Abstract
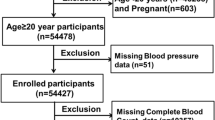
The development of left ventricular hypertrophy (LVH) induced by hypertension is considered a poor prognosis for patients. Similarly, high values of the systemic immune-inflammation index (SII) are correlated with high mortality and morbidity in cardiovascular events. Within this context, our study aimed to detect the association of SII with LVH caused by hypertension. The study included 150 patients diagnosed with hypertension in total and evaluated them as two separate groups with regard to left ventricular mass index (LVMI), including 56 patients (37.3%) with LVH and 94 patients (62.6%) with non-LVH. SII was calculated as platelet × neutrophil/lymphocyte counts. The SII values regarding the group with LVH were detected remarkably higher than those of the non-LVH group (p < 0.001). Additionally, the SII levels of patients with eccentric and concentric hypertrophy were detected higher than those of the normal ventricular geometry and concentric remodeling groups. About curve analysis of the receiver-operating characteristic (ROC), SII values above 869.5 predicted LVH with a sensitivity of 82.1% and specificity of 86.2% (AUC: 0.861; 95% CI: 0.792–0.930; p < 0.001). LVH can be predicted independently through the use of SII in patients diagnosed with hypertension, which may be a simple and easily calculable marker for judging LVH. Moreover, SII can serve as an accurate determinant for the prediction of LVH, in comparison to NLR and PLR.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
All data generated or analysed during this study are included in this published article.
References
World Health Organization. (2009). Global health risks: mortality and burden of disease attributable to selected major risks. World Health Organization. https://apps.who.int/iris/handle/10665/44203.
Tin LL, Beevers DG, Lip GY. Hypertension, left ventricular hypertrophy, and sudden death. Curr Cardiol Rep. 2002;4:449–57.
Lavie CJ, Milani RV, Shah SB, Gilliland YE, Bernal JA, Dinshaw H, et al. Impact of left ventricular geometry on prognosis-a review of ochsner studies. Ochsner J. 2008;8:11–7.
Verdecchia P, Angeli F, Achilli P, Castellani C, Broccatelli A, Gattobigio R, et al. Echocardiographic left ventricular hypertrophy in hypertension: marker for future events or mediator of events? Curr Opin Cardiol. 2007;22:329–34.
Bluemke DA, Kronmal RA, Lima JA, Liu K, Olson J, Burke GL, et al. The relationship of left ventricular mass and geometry to incident cardiovascular events: the MESA (Multi-Ethnic Study of Atherosclerosis) study. J Am Coll Cardiol. 2008;52:2148–55.
Marvar PJ, Thabet SR, Guzik TJ, Lob HE, McCann LA, Weyand C, et al. Central and peripheral mechanisms of T-lymphocyte activation and vascular inflammation produced by angiotensin II-induced hypertension. Circ Res. 2010;107:263–70.
Carreño JE, Apablaza F, Ocaranza MP, Jalil JE. Hipertrofia cardiaca: eventos moleculares y celulares [Cardiac hypertrophy: molecular and cellular events]. Rev Esp Cardiol. 2006;59:473–86. Spanish
Yang R, Chang Q, Meng X, Gao N, Wang W. Prognostic value of Systemic immune-inflammation index in cancer: A meta-analysis. J Cancer. 2018;9:3295–302.
Candemir M, Kiziltunç E, Nurkoç S, Şahinarslan A. Relationship between systemic immune-inflammation index (SII) and the severity of stable coronary artery disease. Angiology. 2021;72:575–81.
Seo M, Yamada T, Morita T, Furukawa Y, Tamaki S, Iwasaki Y, et al. Prognostic value of systemic immune-inflammation index in patients with chronic heart failure. Eur Heart J. 2018;39 suppl 1:P589. https://doi.org/10.1093/eurheartj/ehy564.P589.
Huang J, Zhang Q, Wang R, Ji H, Chen Y, Quan X, et al. Systemic immune-inflammatory index predicts clinical outcomes for elderly patients with acute myocardial infarction receiving percutaneous coronary intervention. Med Sci Monit. 2019;25:9690–701.
Mancia G, Fagard R, Narkiewicz K, Redón J, Zanchetti A, Böhm M, et al. Task Force Members. 2013 ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2013;31:1281–357.
Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39. e14
Virdis A, Dell’Agnello U, Taddei S. Impact of inflammation on vascular disease in hypertension. Maturitas. 2014;78:179–83.
De Miguel C, Rudemiller NP, Abais JM, Mattson DL. Inflammation and hypertension: new understandings and potential therapeutic targets. Curr hypertension Rep. 2015;17:1–10.
Barrows IR, Ramezani A, Raj DS. Inflammation, immunity, and oxidative stress in hypertension—partners in crime? Adv Chronic Kidney Dis. 2019;26:122–30.
Zhang RM, McNerney KP, Riek AE, Bernal‐Mizrachi C. Immunity and hypertension. Acta Physiologica. 2021;231:e13487.
Agita A, Thaha M. Inflammation, immunity, and hypertension. Acta Med Indonesiana. 2017;49:158–65.
Hulsmans M, Sager HB, Roh JD, Valero-Muñoz M, Houstis NE, Iwamoto Y, et al. Cardiac macrophages promote diastolic dysfunction. J Exp Med. 2018;215:423–40.
Guzik TJ, Skiba DS, Touyz RM, Harrison DG. The role of infiltrating immune cells in dysfunctional adipose tissue. Cardiovascular Res. 2017;113:1009–23.
Shankar A, Klein BE, Klein R. Relationship between white blood cell count and incident hypertension. Am J hypertension. 2004;17:233–9.
Bermudez EA, Rifai N, Buring J, Manson JE, Ridker PM. Interrelationships among circulating interleukin-6, C-reactive protein, and traditional cardiovascular risk factors in women. Arteriosclerosis, Thrombosis, Vasc Biol. 2002;22:1668–73.
Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, et al. Role of the T cell in the genesis of angiotensin II–induced hypertension and vascular dysfunction. The. J Exp Med. 2007;204:2449–60.
Abais-Battad JM, Lund H, Fehrenbach DJ, Dasinger JH, Mattson DL. Rag1-null Dahl SS rats reveal that adaptive immune mechanisms exacerbate high protein-induced hypertension and renal injury. Am J Physiol-Regulatory, Integr Comp Physiol. 2018;315:R28–35.
Chan CT, Sobey CG, Lieu M, Ferens D, Kett MM, Diep H, et al. Obligatory role for B cells in the development of angiotensin II–dependent hypertension. Hypertension. 2015;66:1023–33.
Wenzel P, Knorr M, Kossmann S, Stratmann J, Hausding M, Schuhmacher S, et al. Lysozyme M–positive monocytes mediate angiotensin II–induced arterial hypertension and vascular dysfunction. Circulation. 2011;124:1370–81.
Krishnan SM, Ling YH, Huuskes BM, Ferens DM, Saini N, Chan CT, et al. Pharmacological inhibition of the NLRP3 inflammasome reduces blood pressure, renal damage, and dysfunction in salt-sensitive hypertension. Cardiovascular Res. 2019;115:776–87.
Pedrinelli R, Dell’Omo G, Di Bello V, Pellegrini G, Pucci L, Del Prato S, et al. Low-grade inflammation and microalbuminuria in hypertension. Arteriosclerosis, Thrombosis, Vasc Biol. 2004;24:2414–9.
Sesso HD, Buring JE, Rifai N, Blake GJ, Gaziano JM, Ridker PM. C-reactive protein and the risk of developing hypertension. Jama. 2003;290:2945–51.
Sunbul M, Gerin F, Durmus E, Kivrak T, Sari I, Tigen K, et al. Neutrophil to lymphocyte and platelet to lymphocyte ratio in patients with dipper versus non-dipper hypertension. Clin Exp Hypertens. 2014;36:217–21.
Afşin A, Asoğlu R, Kurtoğlu E, Kaya H. Neutrophil to lymphocyte ratio as a predictor of left ventricular hypertrophy in patients with newly diagnosed hypertension. J Hypertens Manag. 2019;5:042.
Tsioufis C, Dimitriadis K, Antoniadis D, Stefanadis C, Kallikazaros I. Inter-relationships of microalbuminuria with the other surrogates of the atherosclerotic cardiovascular disease in hypertensive subjects. Am J Hypertension. 2004;17:470–6.
Lieb W, Mayer B, Stritzke J, Doering A, Hense H-W, Loewel H, et al. Association of low-grade urinary albumin excretion with left ventricular hypertrophy in the general population: the MONICA/KORA Augsburg Echocardiographic Substudy. Nephrol Dialysis Transplant. 2006;21:2780–7.
Hu B, Yang X-R, Xu Y, Sun Y-F, Sun C, Guo W, et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res. 2014;20:6212–22.
Yang YL, Wu CH, Hsu PF, Chen SC, Huang SS, Chan WL, et al. Systemic immune‐inflammation index (SII) predicted clinical outcome in patients with coronary artery disease. Eur J Clin Investig. 2020;50:e13230.
Erdoğan M, Erdöl MA, Öztürk S, Durmaz T. Systemic immune-inflammation index is a novel marker to predict functionally significant coronary artery stenosis. Biomark Med. 2020;14:1553–61.
Dolu AK, Karayiğit O, Ozkan C, Çelik MC, Kalçık M. Relationship between intracoronary thrombus burden and systemic immune-inflammation index in patients with ST-segment elevation myocardial infarction. Acta Cardiol. 2022:1–8.
Ruilope LM, Schmieder RE. Left ventricular hypertrophy and clinical outcomes in hypertensive patients. Am J Hypertens. 2008;21:500–8.
Author information
Authors and Affiliations
Contributions
Concept: OK, SGN; design: OK, MCÇ; supervision: OK, SGN, MCÇ; materials: OK, SGN; data collection and/or processing: OK, SGN; analysis and/or interpretation: MCÇ, OK; literature search: OK, MCÇ, SGN; writing: OK, MCÇ; critical review: MCÇ, OK.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
KARAYİĞİT, O., NURKOÇ, S.G. & Çelik, M.C. Systemic immune-inflammation index (SII) may be an effective indicator in predicting the left ventricular hypertrophy for patients diagnosed with hypertension. J Hum Hypertens 37, 379–385 (2023). https://doi.org/10.1038/s41371-022-00755-0
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41371-022-00755-0
This article is cited by
-
Development and validation of a risk nomogram for predicting recurrence in patients with non-valvular atrial fibrillation after radiofrequency catheter ablation
BMC Medical Informatics and Decision Making (2026)
-
Association between novel Immune-Inflammatory markers and hypertension patients with coronary heart disease: A Cross-Sectional study based on NHANES (2005–2016)
BMC Cardiovascular Disorders (2025)
-
Association between systemic inflammation markers and blood pressure among children and adolescents: National Health and Nutrition Examination Survey
Pediatric Research (2025)
-
Dapagliflozin, inflammation and left ventricular remodelling in patients with type 2 diabetes and left ventricular hypertrophy
BMC Cardiovascular Disorders (2024)
-
Saturation effects of the relationship between physical exercise and systemic immune inflammation index in the short-sleep population: a cross-sectional study
BMC Public Health (2024)