Abstract
Accurate and convenient monitoring of blood pressure (BP) is challenging and relies on cuff-based devices or in the postoperative/intensive care settings, on invasive measurements. The aim of this study was to prospectively evaluate the accuracy of BP measurements obtained from a novel, commercially available cuffless, non-invasive photoplethysmography (PPG)-based chest patch monitor in patients after cardiac surgery. This single center prospective preliminary validation study enrolled adults who underwent cardiac surgery. Data generated by the PPG-based device was compared to those of a standard invasive arterial pressure (IAP). Bland-Altman plots and Pearson’s correlations were used to assess the accuracy of the PPG-based device. Stability and BP changes were not formally evaluated. Ninety-six patients consented for the study. Mean age was 63.2 ± 12.2 years (range 24–84), and 32 (33%) were women. Average monitoring was 25.6 ± 17.2 h. In total, we evaluated 78,659 readings for systolic BP (SBP), 78,818 for diastolic BP (DBP), and 92,544 for heart rate (HR). The correlation coefficients were r = 0.959, 0.973, 0.966, and 0.962 for SBP, DBP, mean arterial pressure (MAP), and (HR), respectively. The bias ± SD was 0.1 ± 4.8 mmHg for SBP; 0.4 ± 2.1 mmHg for DBP; 0.26 ± 2.6 mmHg for MAP, and 0.15 ± 3.6 beats per minutes for HR. 95% of SBP, and 99.9% of DBP measurements were within 10 mmHg of the reference measurement. In conclusion, the tested cuffless device offers acceptable accuracy and is a promising novel noninvasive tool for continuous BP monitoring. Further studies are needed to validate these findings according to the most updated validation protocols for pulseless devices.
ClinicalTrials.gov ID NCT04071015.

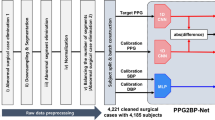
Demonstrating (A) patient’s flow chart; (B) Photoplethysmography sensor principals of action; (C) Study design, parallel measurement of IAP and cuffless device for patients in ICCU; and (D) the main results of the study.Blood pressure (BP), Systolic blood pressure (SBP), Diastolic blood pressure (DBP), Mean arterial pressure (MAP), and Heart rate (HR).
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Data underlying the article will be shared at reasonable request to the corresponding author.
References
Guzik A, Bushnell C. Stroke epidemiology and risk factor management. Continuum. 2017;23:15–39.
Major RH. The history of taking the blood pressure. Ann Med Hist. 1930;2:47–55.
Hu JR, Martin G, Iyengar S, Kovell LC, Plante TB, Helmond NV, et al. Validating cuffless continuous blood pressure monitoring devices. Cardiovasc Digit Health J. 2023;4:9–20.
Sola J, Cortes M, Perruchoud D, De Marco B, Lobo MD, Pellaton C, et al. Guidance for the interpretation of continual cuffless blood pressure data for the diagnosis and management of hypertension. Front Med Technol. 2022;4:899143.
Administration FaD. Noninvasive Blood Pressure Measurement System - Biobeat Platform, BB-613WP Patch - FDA approval. Food and Drug Administration. March 2022. Accessed on March 1, 2025. https://www.accessdata.fda.gov/cdrh_docs/pdf21/K212153.pdf.
Administration FaD. Noninvasive Blood Pressure Measurement System - LiveMetric, LiveOne - FDA approval. Food and Drug Administration, 2020. Accessed on March 1, 2025. https://www.accessdata.fda.gov/cdrh_docs/pdf20/K201302.pdf.
Kario K, Williams B, Tomitani N, McManus RJ, Schutte AE, Avolio A, et al. Innovations in blood pressure measurement and reporting technology: international society of hypertension position paper endorsed by the world hypertension league, European society of hypertension, Asian pacific society of hypertension, and latin American society of hypertension. J Hypertens. 2024;42:1874–88.
Pecchioli V, Lomartire N, Valente L, Pecchioli L, Germanò GI. Comparison study for 24h ambulatory blood pressure measurement (abpm) with biobeat patch abpm® cuffless wearable device in subjects with different body mass index. J Hypertension. 2023;41:E247–E248.
Kachel E, Constantini K, Nachman D, Carasso S, Littman R, Eisenkraft A, et al. A Pilot study of blood pressure monitoring after cardiac surgery using a wearable, non-invasive sensor. Front Med. 2021;8:693926.
Harvey VM, Alexis A, Okeke CAV, McKinley-Grant L, Taylor SC, Desai SR, et al. Integrating skin color assessments into clinical practice and research: a review of current approaches. J Am Acad Dermatol. 2024;91:1189–98.
Performance Evaluation of Pulse Oximeters Taking into Consideration Skin Pigmentation, Race and Ethnicity. United States Food and Drug Administration, 2024. Accessed on March 1, 2025. https://www.fda.gov/media/175828/download.
Saugel B, Kouz K, Meidert AS, Schulte-Uentrop L, Romagnoli S. How to measure blood pressure using an arterial catheter: a systematic 5-step approach. Crit Care. 2020;24:172.
FDA. Biobeat Platform-2. Accessed on March 1, 2025. https://www.accessdata.fda.gov/cdrh_docs/pdf22/K222010.pdf.
Dvir A, Goldstein N, Rapoport A, Balmor RG, Nachman D, Merin R, et al. Comparing cardiac output measurements using a wearable, wireless, noninvasive photoplethysmography-based device to pulse contour cardiac output in the general icu: a brief report. Crit Care Explor. 2022;4:e0624.
Halberthal M, Nachman D, Eisenkraft A, Jaffe E. Hospital and home remote patient monitoring during the COVID-19 outbreak: a novel concept implemented. Am J Disaster Med. 2020;15:149–51.
Nachman D, Eisenkraft A, Goldstein N, Ben-Ishay A, Fons M, Merin R, et al. Influence of sex, bmi, and skin color on the accuracy of non-invasive cuffless photoplethysmography-based blood pressure measurements. Front Physiol. 2022;13:911544.
Nachman D, Gilan A, Goldstein N, Constantini K, Littman R, Eisenkraft A, et al. Twenty-four-hour ambulatory blood pressure measurement using a novel noninvasive, cuffless, wireless device. Am J Hypertens. 2021;34:1171–80.
ISO 81060-3:2022. Non-invasive sphygmomanometers; Part 3: Clinical investigation of continuous automated measurement type. December 2022 (edition 1). https://www.iso.org/standard/71161.html.
Lee H, Park S, Kwon H, Cho B, Park JH, Lee HY. Feasibility and effectiveness of a ring-type blood pressure measurement device compared with 24 h ambulatory blood pressure monitoring device. Korean Circ J. 2024;54:93–104.
Kim J, Chang SA, Park SW. First-in-human study for evaluating the accuracy of smart ring based cuffless blood pressure measurement. J Korean Med Sci. 2024;39:e18.
Hove C, Saeter FW, Stepanov A, Botker-Rasmussen KG, Seeberg TM, Westgaard E, et al. A prototype photoplethysmography-based cuffless device shows promising results in tracking changes in blood pressure. Front Med Technol. 2024;6:1464473.
Tan I, Gnanenthiran SR, Chan J, Kyriakoulis KG, Schlaich MP, Rodgers A, et al. Evaluation of the ability of a commercially available cuffless wearable device to track blood pressure changes. J Hypertens. 2023;41:1003–10.
McGillion MH, Dvirnik N, Yang S, Belley-Cote E, Lamy A, Whitlock R, et al. Continuous noninvasive remote automated blood pressure monitoring with novel wearable technology: a preliminary validation study. JMIR Mhealth Uhealth. 2022;10:e24916.
Nachman D, Gepner Y, Goldstein N, Kabakov E, Ishay AB, Littman R, et al. Comparing blood pressure measurements between a photoplethysmography-based and a standard cuff-based manometry device. Sci Rep. 2020;10:16116.
Islam SMS, Chow CK, Daryabeygikhotbehsara R, Subedi N, Rawstorn J, Tegegne T, et al. Wearable cuffless blood pressure monitoring devices: a systematic review and meta-analysis. Eur Heart J Digit Health. 2022;3:323–37.
Stergiou GS, Avolio AP, Palatini P, Kyriakoulis KG, Schutte AE, Mieke S, et al. European Society of hypertension recommendations for the validation of cuffless blood pressure measuring devices: european society of hypertension working group on blood pressure monitoring and cardiovascular variability. J Hypertens. 2023;41:2074–87.
Stergiou GS, Alpert B, Mieke S, Asmar R, Atkins N, Eckert S, et al. A universal standard for the validation of blood pressure measuring devices: association for the advancement of medical instrumentation/European society of hypertension/international organization for standardization (AAMI/ESH/ISO) collaboration statement. Hypertension. 2018;71:368–74.
Mancia G, Kreutz R, Brunström M, Burnier M, Grassi G, Januszewicz A, et al. 2023 ESH Guidelines for the management of arterial hypertension The Task Force for the management of arterial hypertension of the European society of hypertension: endorsed by the international society of hypertension (ISH) and the European renal association (ERA): erratum. J Hypertens 2024;42:194.
Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the american college of cardiology/american heart association task force on clinical practice guidelines. J Am Coll Cardiol. 2018;71:e127–e248.
Acknowledgements
We thank all staff, nurses and physicians who took part in performing this study as well as patients who consented to the study during the stressful event of heart surgery.
Funding
This study was supported by a grant from Biobeat LTD who provided the pulseless devices and the row data collected by the devices. The company did not have a role in the study design, analysis, interpretation of the results, or writing the manuscript.
Author information
Authors and Affiliations
Contributions
EH and ZZ conceived, designed, wrote, and oversaw all aspects of the study and manuscript preparation. TJ, EZ, and EK were responsible for conducting the study, recruiting participants, and critically reviewing the manuscript. PK and MM performed the statistical analyses and contributed to manuscript review. MDL, LOL, and AL provided critical review and important intellectual input to the manuscript. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
Dr Amir Lerman serves as an advisor to Biobeat. All other co-authors declare no competing interests.
Ethics approval and consent to participate
The study was performed in accordance with the relevant guidelines and regulations. The study protocol was approved by the local institutional review board and registered in ClinicalTrials.gov ID NCT04071015. All participants provided an informed consent to participate in the study. The manuscript does not include identifiable images from human research participants.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hellou, E., Jamal, T., Zuroff, E. et al. Performance of a cuffless photoplethysmography-based device for continuous monitoring of blood pressure after cardiac surgery: a preliminary validation study. J Hum Hypertens 39, 894–902 (2025). https://doi.org/10.1038/s41371-025-01082-w
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41371-025-01082-w