Abstract
Background
Prophylactic indomethacin (3 doses) decreases patent ductus arteriosus (PDA) and intraventricular hemorrhage (IVH) in preterm infants. The study aim was to determine whether single-dose indomethacin (SD-INDO) decreases PDA, IVH, and improves motor function.
Methods
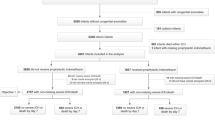
A retrospective cohort (2007–2014) compared infants born < 29 weeks who did (n = 299) or did not (n = 85) receive SD-INDO and estimated outcomes association with ordinal logistic regression, adjusting for multiple variables using propensity scores.
Results
Infants who received SD-INDO were more premature (p < 0.001) but had lower odds of PDA (OR 0.26 [0.15, 0.44], p < 0.005), PDA receiving treatment (OR 0.12 [0.03, 0.47], p < 0.005), death (OR 0.41 [0.20, 0.86], p = 0.02), and CP severity (OR 0.33 [0.12, 0.89], p = 0.03). There was less IVH (OR 0.58 [0.36, 0.94], p = 0.03) when adjusted for gestational age.
Conclusions
SD-INDO is associated with decreased PDA and CP severity and improved survival.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Dani C, Mosca F, Cresi F, Lago P, Lista G, Laforgia N, et al. Patent ductus arteriosus in preterm infants born at 23–24 weeks’ gestation: should we pay more attention? Early Hum Dev. 2019;135:16–22.
Cotton RB, Stahlman MT, Kovar I, Catterton WZ. Medical management of small preterm infants with symptomatic patent ductus arteriosus. J Pediatr. 1978;92:467–73.
Schmidt B, Davis P, Moddemann D, Ohlsson A, Roberts RS, Saigal S, et al. Long-term effects of indomethacin prophylaxis in extremely-low-birth-weight infants. N. Engl J Med. 2001;344:1966–72.
Koch J, Hensley G, Roy L, Brown S, Ramaciotti C, Rosenfeld CR. Prevalence of spontaneous closure of the ductus arteriosus in neonates at a birth weight of 1000 grams or less. Pediatrics 2006;117:1113–21.
Nemerofsky SL, Parravicini E, Bateman D, Kleinman C, Polin RA, Lorenz JM. The ductus arteriosus rarely requires treatment in infants > 1000 grams. Am J Perinatol. 2008;25:661–6.
Clyman RI, Couto J, Murphy GM. Patent ductus arteriosus: are current neonatal treatment options better or worse than no treatment at all? Semin Perinatol. 2012;36:123–9.
Liebowitz M, Clyman RI. Prophylactic indomethacin compared with delayed conservative management of the patent ductus arteriosus in extremely preterm infants: effects on neonatal outcomes. J Pediatr. 2017;187:119–126 e1.
Yoshimoto S, Sakai H, Ueda M, Yoshikata M, Mizobuchi M, Nakao H. Prophylactic indomethacin in extremely premature infants between 23 and 24 weeks gestation. Pediatr Int. 2010;52:374–7.
Bandstra ES, Montalvo BM, Goldberg RN, Pacheco I, Ferrer PL, Flynn J, et al. Prophylactic indomethacin for prevention of intraventricular hemorrhage in premature infants. Pediatrics. 1988;82:533–42.
Ment LR, Oh W, Ehrenkranz RA, Philip AG, Vohr B, Allan W, et al. Low-dose indomethacin and prevention of intraventricular hemorrhage: a multicenter randomized trial. Pediatrics. 1994;93:543–50.
Kluckow M, Jeffery M, Gill A, Evans N. A randomised placebo-controlled trial of early treatment of the patent ductus arteriosus. Arch Dis Child Fetal Neonatal Ed. 2014;99:F99–F104.
Yanowitz TD, Baker RW, Sobchak Brozanski B. Prophylactic indomethacin reduces grades III and IV intraventricular hemorrhages when compared to early indomethacin treatment of a patent ductus arteriosus. J Perinatol. 2003;23:317–22.
Liebowitz M, Koo J, Wickremasinghe A, Allen IE, Clyman RI. Effects of prophylactic indomethacin on vasopressor-dependent hypotension in extremely preterm infants. J Pediatr. 2017;182:21–27 e2.
Gillam-Krakauer M, Hagadorn JI, Reese J. Pharmacological closure of the patent ductus arteriosus: when treatment still makes sense. J Perinatol. 2019;39:1439–41.
Bhat R, Zayek M, Maertens P, Eyal F. A single-dose indomethacin prophylaxis for reducing perinatal brain injury in extremely low birth weight infants: a non-inferiority analysis. J Perinatol. 2019;39:1462–71.
Bada HS, Green RS, Pourcyrous M, Leffler CW, Korones SB, Magill HL, et al. Indomethacin reduces the risks of severe intraventricular hemorrhage. J Pediatr. 1989;115:631–7.
Ment LR, Duncan CC, Ehrenkranz RA, Kleinman CS, Pitt BR, Taylor KJ, et al. Randomized indomethacin trial for prevention of intraventricular hemorrhage in very low birth weight infants. J Pediatr. 1985;107:937–43.
Ment LR, Duncan CC, Ehrenkranz RA, Kleinman CS, Taylor KJ, Scott DT, et al. Randomized low-dose indomethacin trial for prevention of intraventricular hemorrhage in very low birth weight neonates. J Pediatr. 1988;112:948–55.
Laptook AR, O’Shea TM, Shankaran S, Bhaskar B, Network NN. Adverse neurodevelopmental outcomes among extremely low birth weight infants with a normal head ultrasound: prevalence and antecedents. Pediatrics. 2005;115:673–80.
Wood NS, Costeloe K, Gibson AT, Hennessy EM, Marlow N, Wilkinson AR, et al. The EPICure study: associations and antecedents of neurological and developmental disability at 30 months of age following extremely preterm birth. Arch Dis Child Fetal Neonatal Ed. 2005;90:F134–40.
Ment LR, Vohr B, Allan W, Westerveld M, Sparrow SS, Schneider KC, et al. Outcome of children in the indomethacin intraventricular hemorrhage prevention trial. Pediatrics. 2000;105:485–91.
Williams J, Lee KJ, Anderson PJ. Prevalence of motor-skill impairment in preterm children who do not develop cerebral palsy: a systematic review. Dev Med Child Neurol. 2010;52:232–7.
Krueger E, Mellander M, Bratton D, Cotton R. Prevention of symptomatic patent ductus arteriosus with a single dose of indomethacin. J Pediatr. 1987;111:749–54.
Papile LA, Munsick-Bruno G, Schaefer A. Relationship of cerebral intraventricular hemorrhage and early childhood neurologic handicaps. J Pediatr. 1983;103:273–7.
Gordon PV. Understanding intestinal vulnerability to perforation in the extremely low birth weight infant. Pediatr Res. 2009;65:138–44.
Lin PW, Stoll BJ. Necrotising enterocolitis. Lancet. 2006;368:1271–83.
Selewski DT, Charlton JR, Jetton JG, Guillet R, Mhanna MJ, Askenazi DJ, et al. Neonatal acute kidney injury. Pediatrics. 2015;136:e463–73.
Parry G, Tucker J, Tarnow-Mordi W, Group UKNSSC. CRIB II: an update of the clinical risk index for babies score. Lancet. 2003;361:1789–91.
Richardson DK, Corcoran JD, Escobar GJ, Lee SK. SNAP-II and SNAPPE-II: simplified newborn illness severity and mortality risk scores. J Pediatr. 2001;138:92–100.
Albers CA, Grieve AJ. Review of Bayley Scales of infant and toddler development - third edition. J Psychoed Assoc. 2007;25:180–90.
Kuban KC, Allred EN, O’Shea M, Paneth N, Pagano M, Leviton A, et al. An algorithm for identifying and classifying cerebral palsy in young children. J Pediatr. 2008;153:466–72.
Palisano RJ, Rosenbaum P, Bartlett D, Livingston MH. Content validity of the expanded and revised Gross Motor Function Classification System. Dev Med Child Neurol. 2008;50:744–50.
McCullagh P. Regression models for ordinal data. J R Stat Soc Ser B. 1980;42:109–42.
Gamaleldin I, Harding D, Siassakos D, Draycott T, Odd D. Significant intraventricular hemorrhage is more likely in very preterm infants born by vaginal delivery: a multi-centre retrospective cohort study. J Matern Fetal Neonatal Med. 2019;32:477–82.
Humberg A, Hartel C, Paul P, Hanke K, Bossung V, Hartz A, et al. Delivery mode and intraventricular hemorrhage risk in very-low-birth-weight infants: observational data of the German Neonatal Network. Eur J Obstet Gynecol Reprod Biol. 2017;212:144–9.
Shankaran S, Lin A, Maller-Kesselman J, Zhang H, O’Shea TM, Bada HS, et al. Maternal race, demography, and health care disparities impact risk for intraventricular hemorrhage in preterm neonates. J Pediatr. 2014;164:1005–1011 e3.
Rosenbaum P, Rubin D. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55.
Gelfond JA, Heitman E, Pollock BH, Klugman CM. Principles for the ethical analysis of clinical and translational research. Stat Med. 2011;30:2785–92.
Thalji AA, Carr I, Yeh TF, Raval D, Luken JA, Pildes RS. Pharmacokinetics of intravenously administered indomethacin in premature infants. J Pediatr. 1980;97:995–1000.
Yaffe SJ, Friedman WF, Rogers D, Lang P, Ragni M, Saccar C. The disposition of indomethacin in preterm babies. J Pediatr. 1980;97:1001–6.
Alpert BS, Lewins MJ, Rowland DW, Grant MJ, Olley PM, Soldin SJ, et al. Plasma indomethacin levels in preterm newborn infants with symptomatic patent ductus arteriosus-clinical and echocardiographic assessments of response. J Pediatr. 1979;95:578–82.
Brash AR, Hickey DE, Graham TP, Stahlman MT, Oates JA, Cotton RB. Pharmacokinetics of indomethacin in the neonate. Relation of plasma indomethacin levels to response of the ductus arteriosus. N. Engl J Med. 1981;305:67–72.
Wiest DB, Pinson JB, Gal PS, Brundage RC, Schall S, Ransom JL, et al. Population pharmacokinetics of intravenous indomethacin in neonates with symptomatic patent ductus arteriosus. Clin Pharm Ther. 1991;49:550–7.
Vert P, Bianchetti G, Marchal F, Monin P, Morselli PL. Effectiveness and pharmacokinetics of indomethacin in premature newborns with patent ductus arteriosus. Eur J Clin Pharm. 1980;18:83–8.
Ment LR, Vohr BR, Makuch RW, Westerveld M, Katz KH, Schneider KC, et al. Prevention of intraventricular hemorrhage by indomethacin in male preterm infants. J Pediatr. 2004;145:832–4.
Schmidt B, Roberts RS, Anderson PJ, Asztalos EV, Costantini L, Davis PG, et al. Academic performance, motor function, and behavior 11 years after neonatal caffeine citrate therapy for apnea of prematurity: an 11-year follow-up of the CAP randomized clinical trial. JAMA Pediatrics. 2017;171:564–72.
Spittle AJ, Cheong J, Doyle LW, Roberts G, Lee KJ, Lim J, et al. Neonatal white matter abnormality predicts childhood motor impairment in very preterm children. Dev Med Child Neurol. 2011;53:1000–6.
Miller SP, Mayer EE, Clyman RI, Glidden DV, Hamrick SE, Barkovich AJ. Prolonged indomethacin exposure is associated with decreased white matter injury detected with magnetic resonance imaging in premature newborns at 24 to 28 weeks’ gestation at birth. Pediatrics 2006;117:1626–31.
Norton ME, Merrill J, Cooper BA, Kuller JA, Clyman RI. Neonatal complications after the administration of indomethacin for preterm labor. N. Engl J Med. 1993;329:1602–7.
Watson A, Saville B, Lu Z, Walsh W. It is not the ride: inter-hospital transport is not an independent risk factor for intraventricular hemorrhage among very low birth weight infants. J Perinatol. 2013;33:366–70.
Jensen EA, Dysart KC, Gantz MG, Carper B, Higgins RD, Keszler M, et al. Association between use of prophylactic indomethacin and the risk for bronchopulmonary dysplasia in extremely preterm infants. J Pediatr. 2017;186:34–40 e2.
Semberova J, Sirc J, Miletin J, Kucera J, Berka I, Sebkova S, et al. Spontaneous closure of patent ductus arteriosus in infants < /=1500 g. Pediatrics. 2017;140:e20164258.
Mirza H, Laptook AR, Oh W, Vohr BR, Stoll BJ, Kandefer S, et al. Effects of indomethacin prophylaxis timing on intraventricular haemorrhage and patent ductus arteriosus in extremely low birth weight infants. Arch Dis Child Fetal Neonatal Ed. 2016;101:F418–22.
Clyman RI, Chorne N. Patent ductus arteriosus: evidence for and against treatment. J Pediatr. 2007;150:216–9.
Noori S, Seri I. Hemodynamic antecedents of peri/intraventricular hemorrhage in very preterm neonates. Semin Fetal Neonatal Med. 2015;20:232–7.
Brooks JM, Travadi JN, Patole SK, Doherty DA, Simmer K. Is surgical ligation of patent ductus arteriosus necessary? The Western Australian experience of conservative management. Arch Dis Child Fetal Neonatal Ed. 2005;90:F235–9.
Kaempf JW, Wu YX, Kaempf AJ, Kaempf AM, Wang L, Grunkemeier G. What happens when the patent ductus arteriosus is treated less aggressively in very low birth weight infants? J Perinatol. 2012;32:344–8.
Hagadorn JI, Bennett MV, Brownell EA, Payton KSE, Benitz WE, Lee HC. Covariation of neonatal intensive care unit-level patent ductus arteriosus management and in-neonatal intensive care unit outcomes following preterm birth. J Pediatr. 2018;203:225–233 e1.
Hagadorn JI, Brownell EA, Trzaski JM, Johnson KR, Lainwala S, Campbell BT, et al. Trends and variation in management and outcomes of very low-birth-weight infants with patent ductus arteriosus. Pediatr Res. 2016;80:785–92.
Jensen EA, Foglia EE, Schmidt B. Association between prophylactic indomethacin and death or bronchopulmonary dysplasia: a systematic review and meta-analysis of observational studies. Semin Perinatol. 2018;42:228–34.
Kaempf J, Huston R, Wu YX, Kaempf AJ, Wang L, Grunkemeier G, et al. Permissive tolerance of the patent ductus arteriosus may increase the risk of chronic lung disease. Res Rep Neonatol. 2013;3:5–10.
Sadeck LS, Leone CR, Procianoy RS, Guinsburg R, Marba ST, Martinez FE, et al. Effects of therapeutic approach on the neonatal evolution of very low birth weight infants with patent ductus arteriosus. J Pediatr. 2014;90:616–23.
Farooqui MA, Elsayed YN, Jeyaraman MM, Dingwall O, Tagin M, Zarychanski R, et al. Pre-symptomatic targeted treatment of patent ductus arteriosus in preterm newborns: a systematic review and meta-analysis. J Neonatal Perinat Med. 2019;12:1–7.
Jetton JG, Askenazi DJ. Update on acute kidney injury in the neonate. Curr Opin Pediatr. 2012;24:191–6.
Funding
Vanderbilt Institute for Clinical and Translational Research grant support (UL1 TR000445 from NCATS/NIH) (MGK) and National Institutes of Health HL109199 and HL128386 (JR).
Author information
Authors and Affiliations
Contributions
MGK designed the study, performed the data collection, and wrote and revised the manuscript. RBC designed the study. CS performed statistical analysis. BR performed data collection. JR designed the study and revised the manuscript. NM designed the study, performed the data collection and performed statistical analysis.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics
The authors affirm that research was conducted in accordance with the ethical standards of all applicable national and institutional committees and the World Medical Association’s Helsinki Declaration.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Gillam-Krakauer, M., Slaughter, J.C., Cotton, R.B. et al. Outcomes in infants < 29 weeks of gestation following single-dose prophylactic indomethacin. J Perinatol 41, 109–118 (2021). https://doi.org/10.1038/s41372-020-00814-9
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41372-020-00814-9
This article is cited by
-
A FAR-Out approach for evaluating the impact of clinical practice changes on severe intracranial hemorrhage in preterm infants
Journal of Perinatology (2026)
-
Response of the ductus arteriosus to acetaminophen or indomethacin in extremely low birth weight infants
Journal of Perinatology (2025)
-
Neuroprotection care bundle implementation is associated with improved long-term neurodevelopmental outcomes in extremely premature infants
Journal of Perinatology (2022)