Abstract
Background
Randomized trials of antenatal steroids (ANS) included women at 24–33 weeks gestational age (GA); however, few women had preeclampsia and women with diabetes mellitus (DM) were excluded.
Methods
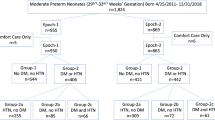
Cohort study including preterm births at 230/7–286/7 weeks GA before (Epoch-1) and after (Epoch-2) expansion of ANS administration to women with DM and hypertensive disorders (HTN). We compared Group-A (neither DM nor HTN) and Group-B (DM and/or HTN).
Results
Among 747 neonates the adjusted odds ratio (aOR) for surfactant administration, in-hospital mortality, severe intraventricular hemorrhage (IVH) and death or severe IVH were lower in ANS-exposed neonates than unexposed neonates. In Group-B, ANS administration was independently associated with less severe IVH and less death or severe IVH, but not less surfactant use or mortality.
Conclusions
Increased ANS administration in women with DM and/or HTN was independently associated with less severe IVH and less death or severe IVH but without decrease in surfactant administration.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
McGoldrick E, Stewart F, Parker R, Dalziel SR. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2020, Issue 12. Art. No.: CD004454. Accessed 13 March 2021.
WHO ACTION Trials Collaborators. The World Health Organization ACTION-I (Antenatal Corticosteroids for Improving Outcomes in preterm Newborns) Trial: a multi-country, multi-centre, two-arm, parallel, double-blind, placebo-controlled, individually randomized trial of antenatal corticosteroids for women at risk of imminent birth in the early preterm period in hospitals in low-resource countries. Trials 2019;20:507.
Schmitz T, Alberti C, Ursino M, Baud O, Aupiais C. BETADOSE study group and the GROG (Groupe de Recherche en Gynécologie Obstétrique). Full versus half dose of antenatal betamethasone to prevent severe neonatal respiratory distress syndrome associated with preterm birth: study protocol for a randomised, multicenter, double blind, placebo-controlled, non-inferiority trial (BETADOSE). BMC Pregnancy Childbirth. 2019;19:67.
Melamed N, Shah J, Soraisham A, Yoon EW, Lee SK, Shah PS, et al. Association Between Antenatal Corticosteroid Administration-to-Birth Interval and Outcomes of Preterm Neonates. Obstet Gynecol. 2015;125:1377–84.
Bloom SL, Leveno KJ. Corticosteroid use in special circumstances: preterm ruptured membranes, hypertension, fetal growth restriction, multiple fetuses. Clin Obstetr Gynecol. 2003;46:150–60.
Amorim MMR, Santos LC, Faúndes A. Corticosteroid therapy for prevention of respiratory distress syndrome in severe preeclampsia. Am J Obstet Gynecol. 1999;180:1283–8.
Sheehan JW, Pritchard M, Heyne RJ, Brown LS, Jaleel MA, Engle WD, et al. Severe intraventricular hemorrhage and withdrawal of support in preterm infants. J Perinatol. 2017;37:441–7.
Stoll BJ, Hansen NI, Bell EF, Shankaran S, Laptook AR, Walsh MC, et al. Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics. 2010;126:443–56.
Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr. 1978;92:529–34.
Dykes FD, Lazzara A, Ahmann P, Blumenstein B, Schwartz J, Brann AW. Intraventricular hemorrhage: a prospective evaluation of etiopathogenesis. Pediatrics. 1980;66:42–9.
Schmidt B, Seshia M, Shankaran S, Mildenhall L, Tyson J, Lui K, et al. Trial of Indomethacin Prophylaxis in Preterms Investigators. Effects of prophylactic indomethacin in extremely low-birth-weight infants with and without adequate exposure to antenatal corticosteroids. Arch Pediatr Adolesc Med. 2011;165:642–6.
Schmidt B, Davis P, Moddemann D, Ohlsson A, Roberts RS, Saigal S, et al. Trial of Indomethacin Prophylaxis in Preterms Investigators. Long-term effects of indomethacin prophylaxis in extremely-low-birth-weight infants. N Engl J Med. 2001;344:1966–72.
Sarkar S, Bhagat I, Dechert R, Schumacher RE, Donn SM. Severe intraventricular hemorrhage in preterm infants: comparison of risk factors and short-term neonatal morbidities between grade 3 and grade 4 intraventricular hemorrhage. Am J Perinatol. 2009;26:419–24.
Stoll BJ, Hansen NI, Bell EF, Walsh MC, Carlo WA, Shankaran S, et al. Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Trends in Care Practices, Morbidity, and Mortality of Extremely Preterm Neonates, 1993-2012. JAMA. 2015;8:1039–51.
Lim J, Hagen E. Reducing Germinal Matrix-Intraventricular Hemorrhage: perinatal and Delivery Room Factors. Neoreviews. 2019;20:e452–e463.
Kakkilaya V, Jubran I, Mashruwala V, Ramon E, Simcik VN, Marshall M, et al. Quality Improvement Project to Decrease Delivery Room Intubations in Preterm Infants. Pediatrics. 2019;143:pii: e20180201.
Sanchez AMMS, Jimenez JM, Manroe BL, Rosenfeld CR, Tyson TE. Systems approach to the evaluation of maternal and neonatal care. Proc 12th Hawaii Int’l Conf Syst Sci. 1979;III:140151. Selected Papers in Medical Information Processing.
Arnautovic TI, Longo JL, Trail-Burns EJ, Tucker R, Keszler M, Laptook AR. Antenatal Risk Factors Associated with Spontaneous Intestinal Perforation in Preterm Infants Receiving Post-natal Indomethacin. J Pediatr. 2021. https://doi.org/10.1016/j.jpeds.2021.01.011.
Olsen IE, Groveman SA, Lawson ML, Clark RH, Zemel BS. New intrauterine growth curves based on United States data. Pediatrics. 2010;125:e214–e224.
McIntire DD, Bloom SL, Casey BM, Leveno KJ. Birth weight in relation to morbidity and mortality among newborn infants. N Engl J Med. 1999;340:1234–8.
Peduzzi P, Concato J, Kemper E, Holford TR, Feinstein AR. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol. 1996;49:1373–9.
Schreiber-Gregory D. Multicollinearity: What Is It, Why Should We Care, and How Can It Be Controlled? Paper 1404-2017. http://support.sas.com/resources/papers/proceedings17/1404-2017.pdf, accessed 2/20/21.
Chawla S, Natarajan G, Chowdhury D, Das A, Walsh M, Bell EF, et al. Neonatal Morbidities among Moderately Preterm Infants with and without Exposure to Antenatal Corticosteroids. Am J Perinatol. 2018;35:1213–21.
Norman M, Piedvache A, Børch K, Huusom LD, Bonamy AE, Howell EA, et al. Effective Perinatal Intensive Care in Europe (EPICE) Research Group. Association of Short Antenatal Corticosteroid Administration-to-Birth Intervals With Survival and Morbidity Among Very Preterm Infants: results From the EPICE Cohort. JAMA Pediatr. 2017;171:678–86.
Norberg H, Kowalski J, Maršál K, Norman M. Timing of antenatal corticosteroid administration and survival in extremely preterm infants: a national population-based cohort study. BJOG. 2017;124:1567–74.
Battarbee AN, Sandoval G, Grobman WA, Bailit JL, Reddy UM, Wapner RJ, et al. Eunice Kennedy Shriver National Institute of Child Health Human Development Maternal-Fetal Medicine Units (MFMU) Network. Antenatal Corticosteroids and Preterm Neonatal Morbidity and Mortality among Women with and without Diabetes in Pregnancy. Am J Perinatol. 2020 Jul 27:https://doi.org/10.1055/s-0040-1714391.
Collaborative Group on Antenatal Steroid Therapy. Effect of antenatal dexamethasone administration on the prevention of respiratory distress syndrome. Am J Obstetr Gynecol. 1981;141:276–87.
Fekih M, Chaieb A, Sboui H, DenguezliW, Hidar S, Khairi H. Value of prenatal corticotherapy in the prevention of hyaline membrane disease in premature infants. Randomized prospective study [Apport de la corticothérapie antenatale dans la prevention de la maladie des membranes hyalines chez le prématuré. Etude prospective randomisée]. Tunis Médicale. 2002;80:260–5.
Liggins GC, Howie RN. A controlled trial of antepartum glucocorticoid treatment for prevention of the respiratory distress syndrome in premature infants. Pediatrics. 1972;50:515–25.
Gamsu HR, Mullinger BM, Donnai P, Dash CH. Antenatal administration of betamethasone to prevent respiratory distress syndrome in preterm infants: report of a UK multicentre trial. BR J Obstetr Gynaecol. 1989;96:401–10.
Morsing E, Maršál K, Ley D. Reduced Prevalence of Severe Intraventricular Hemorrhage in Very Preterm Infants Delivered after Maternal Preeclampsia. Neonatology. 2018;114:205–11.
Shankaran S, Lin A, Maller-Kesselman J, Zhang H, O’Shea TM, Bada HS, et al. Gene Targets for Intraventricular Hemorrhage Study. Maternal race, demography, and health care disparities impact risk for intraventricular hemorrhage in preterm neonates. J Pediatr. 2014;164:1005–11.
Aufdenblatten M, Baumann M, Raio L, Dick B, Frey BM, Schneider H, et al. Prematurity is related to high placental cortisol in preeclampsia. Pediatr Res. 2009;65:198–202.
Sen S, Reghu A, Ferguson SD. Efficacy of a single dose of antenatal steroid in surfactant-treated babies under 31weeks’ gestation. J Matern Fetal Neonatal Med. 2002;12:298–303.
Elgendy MM, Othman HF, Heis F, Qattea I, Aly H. Spontaneous intestinal perforation in premature infants: a national study. J Perinatol. 2021 Mar 5. https://doi.org/10.1038/s41372-021-00990-2.
Kaiser JR, Tilford JM, Simpson PM, Salhab WA, Rosenfeld CR. Hospital survival of very-low-birth-weight neonates from 1977 to 2000. J Perinatol. 2004;24:343–50.
Acknowledgements
We thank Dr Bloom, Dr Leveno, Dr McIntire, Dr Pérez-Fontán and Dr Savani for participating in the discussions that eventually led to expansion of ANS to mothers with DM and HTN on 9/21/2015. Preliminary results were presented at the following meetings:
1. HMW, MAJ, MF, LPB. Parkland QI Bundle: Reducing Mortality and Morbidity in Extremely Low Gestational Age Neonates (ELGANs) at Parkland. Poster presentation at AAP National Conference and Exhibition, Washington DC, 10/23/2015.
2. HMW, MAJ, MSF, PJB, LPB. A Quality Improvement (QI) Project to Reduce Severe Intraventricular Hemorrhage (IVH). Poster at Pediatric Academy Societies Meeting, Toronto, Canada, 5/5/2018
3. HMW, MAJ, MSF, PJB, LPB. Interaction between Prophylactic Indomethacin (PIndo) and Antenatal Steroids (ANS) leading to Spontaneous Intestinal Perforation (SIP). Poster at Pediatric Academy Societies Meeting, Toronto, Canada, 5/7/2018.
Funding
No external funding for this paper.
Author information
Authors and Affiliations
Contributions
Dr HMW conceptualized and designed the study, collected data, coordinated and supervised data collection, carried out initial analyses, drafted the initial paper, and reviewed and revised the paper. Drs MAJ, CRR, MSF and LPB conceptualized and designed the study and reviewed and revised the paper. Ms PJB collected data and reviewed and revised the paper. Dr LPB carried out the analyses. All authors approved the final paper as submitted and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Weydig, H.M., Rosenfeld, C.R., Jaleel, M.A. et al. Association of antenatal steroids with neonatal mortality and morbidity in preterm infants born to mothers with diabetes mellitus and hypertension. J Perinatol 41, 1660–1668 (2021). https://doi.org/10.1038/s41372-021-01090-x
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41372-021-01090-x
This article is cited by
-
A validated NICU database: recounting 50 years of clinical growth, quality improvement and research
Pediatric Research (2024)
-
Association of antenatal steroids with surfactant administration in moderate preterm infants born to women with diabetes mellitus and/or hypertension
Journal of Perinatology (2022)