Abstract
Objective
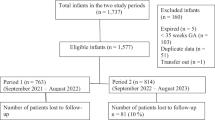
We analyze phototherapy rates after implementation of a Hyperbilirubinemia Clinical Pathway (HCP), which placed full-term ABOi DAT negative newborns on the low risk phototherapy nomogram, rather than medium risk, as previously done.
Study design
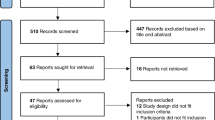
A chart review was performed for ABOi newborns born ≥36 weeks gestation between January 2020 and October 2021. Primary outcome measures were rates of phototherapy across pre- and post-intervention groups and among DAT negative newborns.
Results
There was an increased proportion of newborns assigned to the low risk curve after the intervention. There were no significant differences in phototherapy rates among the intervention groups, although there was a non-significant decrease in phototherapy rates among DAT negative newborns after the intervention. There were no increases in adverse outcomes.
Conclusions
Providers adhered to the guidelines after implementation of the HCP. ABOi DAT negative newborns can be viewed as low risk for hyperbilirubinemia requiring phototherapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
The dataset generated and analyzed during the study are available from the corresponding author on reasonable request.
References
Porter ML, Dennis BL. Hyperbilirubinemia in the term newborn. Am Fam Phys. 2002;65:599–606.
Subcommittee on Hyperbilirubinemia. Management of Hyperbilirubinemia in the Newborn Infant 35 or More Weeks of Gestation. Pediatrics. 2004;114:297–316.
Kuzniewicz MW, Escobar GJ, Newman TB. Impact of universal bilirubin screening on severe hyperbilirubinemia and phototherapy use. Pediatrics. 2009;124:1031–9.
Delaney M, Matthews DC. Hemolytic disease of the fetus and newborn: managing the mother, fetus, and newborn. Hematol Am Soc Hematol Educ Program. 2015;2015:146–51.
Murray NA, Roberts IA. Haemolytic disease of the newborn. Arch Dis Child Fetal Neonatal Ed. 2007;92:F83–8.
Valsami S, Politou M, Boutsikou Τ, Boutsikou T, Briana D, Paptesta M, et al. Importance of Direct Antiglobulin Test (DAT) in Cord Blood: Causes of DAT (+) in a Cohort Study. Pediatr Neonatol. 2015;56:256–60.
Dinesh D. Review of positive direct antiglobulin tests found on cord blood sampling. J Paediatr Child Health. 2005;41:504–7.
Keir A, Agpalo M, Lieberman L, Callum J. How to use: the direct antiglobulin test in newborns. Arch Dis Child Educ Pr Ed. 2015;100:198–203.
Özgönenel B, Kukreja G, O’Malley B, Bluth MH. Neonatal BO Incompatibility Is Associated With a Positive Cord Blood Direct Antiglobulin Test in Infants of Black Ethnicity. J Pediatr Hematol Oncol. 2015;37:e453–7.
Maisels MJ, Bhutani VK, Bogen D, Newman TB, Stark AR, Watchko JF, et al. Hyperbilirubinemia in the Newborn Infant ≥35 Weeks’ Gestation: An Update With Clarifications. Pediatrics. 2009;124:1193–8.
DePorre AG, Hall M, Puls HT, Daly A, Gay JC, Bettenhausen JL, et al. Variation in Care and Clinical Outcomes Among Infants Hospitalized With Hyperbilirubinemia. Hosp Pediatr. 2020;10:844–50.
Romero HM, Ringer C, Leu MG, Beardsley E, Kelly K, Fesinmeyer MD, et al. Neonatal jaundice: improved quality and cost savings after implementation of a standard pathway. Pediatrics. 2018;141:e20161472.
Preloger E, Wedoff M, Lemke JT, Pan A, Nelson A. Decreasing Laboratory Testing for Neonatal Jaundice Through Revision of a Clinical Practice Pathway. Hosp Pediatr. 2022;12:e67–e72.
Kemper AR, Newman TB, Slaughter JL, Maisels MJ, Watchko JF, Downs SM, et al. Clinical Practice Guideline Revision: Management of Hyperbilirubinemia in the Newborn Infant 35 or More Weeks of Gestation. Pediatrics. 2022;150:e2022058859.
Bhutani VK, Gourley GR, Adler S, Kreamer B, Dalin C, Johnson LH. Noninvasive measurement of total serum bilirubin in a multiracial predischarge newborn population to assess the risk of severe hyperbilirubinemia. Pediatrics 2000;106:E17 https://doi.org/10.1542/peds.106.2.e17.
Herschel M, Karrison T, Wen M, Caldarelli L, Baron B. Isoimmunization Is Unlikely to Be the Cause of Hemolysis in ABO-Incompatible but Direct Antiglobulin Test-Negative Neonates. Pediatrics. 2002;110:127–30.
Bhutani VK, Stark AR, Lazzeroni LC, Poland R, Gourley GR, Kazmierczak S, et al. Predischarge screening for severe neonatal hyperbilirubinemia identifies infants who need phototherapy. J Pediatr. 2013;162:477–82.
Keren R, Luan X, Friedman S, Saddlemire S, Cnaan A, Bhutani VK. A comparison of alternative risk-assessment strategies for predicting significant neonatal hyperbilirubinemia in term and near- term infants. Pediatrics. 2008;121:e170–e179.
Kuzniewicz MW, Park J, Niki H, Walsh EM, McCulloch CE, Newman TB. Predicting the Need for Phototherapy After Discharge. Pediatrics. 2021;147:e2020019778.
Chang PW, Kuzniewicz MW, McCulloch CE, Newman TB. A Clinical Prediction Rule for Rebound Hyperbilirubinemia Following Inpatient Phototherapy. Pediatrics. 2017;139:e20162896.
Schutzman DL, Sekhon R, Hundalani S. Hour-specific bilirubin nomogram in infants with ABO incompatibility and direct Coombs-positive results. Arch Pediatr Adolesc Med. 2010;164:1158–64.
Peeters B, Geerts I, Van Mullem M, Micalessi I, Saegeman V, Moerman J. Post-test probability for neonatal hyperbilirubinemia based on umbilical cord blood bilirubin, direct antiglobulin test, and ABO compatibility results. Eur J Pediatr. 2016;175:651–7.
Alshammari S, Alqashami A, Alhumud S, Aladadh M, Alsaif S, Ali K. Neonatal ABO incompatibility, influence of blood group, and coomb’s test on outcome. J Clin Neonatol. 2022;11:212–8.
Ozolek JA, Watchko JF, Mimouni F. Prevalence and lack of clinical significance of blood group incompatibility in mothers with blood type A or B. J Pediatr. 1994;125:87–91.
Wickremasinghe AC, Kuzniewicz MW, McCulloch CE, Newman TB. Efficacy of Subthreshold Newborn Phototherapy During the Birth Hospitalization in Preventing Readmission for Phototherapy. JAMA Pediatrics. 2018;172:378–85.
Frank JE. Diagnosis and management of G6PD deficiency. Am Fam Phys. 2005;72:1277–82.
Acknowledgements
The authors thank Elizabeth Woodard, NP, Ginny Combs, MSN, Cheryl Slater, MSN and Jennifer Pfau, MD, for their assistance with implementation of the Hyperbilirubinemia Clinical Pathway at Boston Medical Center.
Funding
This work was supported by a Fred Lovejoy Resident Research and Education Award and the Urban Health and Advocacy Track Grant.
Author information
Authors and Affiliations
Contributions
Dr JMG conceptualized and designed the study, collected data, drafted the paper and revised the paper. Dr EMA and Mr ST collected data, and reviewed and revised the paper. Mr BMB designed the data collection instruments, carried out analyses, and reviewed and revised the paper. Dr BS designed the study, and reviewed and revised the paper. Dr TG conceptualized and designed the study, coordinated and supervised data collection, and critically reviewed the paper. All authors approved the final paper as submitted and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gabbay, J.M., Agneta, E.M., Turkington, S. et al. Rates of phototherapy among ABO-incompatible newborns with a negative direct antiglobulin test. J Perinatol 43, 1357–1362 (2023). https://doi.org/10.1038/s41372-023-01650-3
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41372-023-01650-3