Abstract
Objectives
(1) To demonstrate feasibility and safety of surfactant administration via laryngeal mask airway (LMA) as a first-line therapy. (2) To measure treatment success, defined as avoidance of intubation/invasive mechanical ventilation, and determine if specific clinical variables could predict success/failure.
Study design
Observational cohort with eligible infants given surfactant using one type of LMA via standardized protocol. Data was captured prospectively followed by retrospective chart review.
Results
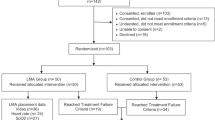
120 infants ≥1250 g and 28.3–41.1 weeks gestation were included. First-line LMA surfactant therapy was successful in 70% of the infants and those infants weaned to room air significantly quicker than infants requiring subsequent intubation/mechanical ventilation (p = 0.002 by 72 h, p = 0.001 by 96 h). Clinical variables assessed could not predict treatment success/failure. Complications were infrequent and did not differ between groups.
Conclusion
First-line LMA surfactant is feasible and safe for certain infants. Prediction of treatment success was not possible in our cohort.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
Data availability
The datasets generated during and analyzed during the current study are not publicly available due to IRB restrictions but are available from the corresponding author on reasonable request.
References
Guthrie SO, Roberts KD. Less invasive surfactant administration methods: who, what and how. J Perinatol. 2023. https://doi.org/10.1038/s41372-023-01778-2.
Guthrie SO, Fort P, Roberts KD. Surfactant administration through laryngeal or supraglottic airways. Neoreviews. 2021;22:e673–88.
Abdel-Latif ME, Osborn DA. Laryngeal mask airway surfactant administration for prevention of morbidity and mortality in preterm infants with or at risk of respiratory distress syndrome. Cochrane Database Syst Rev. 2024;1:CD008309.
Roberts CT, Manley BJ, O’Shea JE, Stark M, Andersen C, et al. Supraglottic airway devices for administration of surfactant to newborn infants with respiratory distress syndrome: a narrative review. Arch Dis Child Fetal Neonatal Ed. 2021;106:336–41.
Calevo MG, Veronese N, Cavallin F, Paola C, Micaglio M, et al. Supraglottic airway devices for surfactant treatment: systematic review and meta-analysis. J Perinatol. 2019;39:173–83.
Singh R, Mohan CVR, Taxak S. Controlled Trial to evaluate the use of LMA for neonatal resuscitation. J Anaesthesiol Clin Pharm. 2005;21:303–6.
Trevisanuto D, Cavallin F, Nguyen LN, Nguyen TV, Tran LD, et al. Supreme laryngeal mask airway versus face mask during neonatal resuscitation: a randomized controlled trial. J Pediatr. 2015;167:286–91.
Zhu X-Y, Lin B-C, Zhang Q-S, Ye H-M, Yu R-J, et al. A prospective evaluation of the efficacy of the laryngeal mask airway during neonatal resuscitation. Resuscitation. 2011;82:1405–9.
Aldana-Aguirre JC, Pinto M, Featherstone RM, Kumar M. Less invasive surfactant administration versus intubation for surfactant delivery in preterm infants with respiratory distress syndrome: a systematic review and meta-analysis. Arch Dis Child Fetal Neonatal Ed. 2017;102:F17–23.
Kanmaz HG, Erdeve O, Canpolat FE, Mutlu B, Dilmen U. Surfactant administration via thin catheter during spontaneous breathing: randomized controlled trial. Pediatrics. 2013;131:e502–9.
Kribs A, Roll C, Göpel W, Weig C, Groneck P, et al. NINSAPP Trial Investigators. Nonintubated surfactant application vs conventional therapy in extremely preterm infants: a randomized clinical trial. JAMA Pediatr. 2015;169:723–30.
Dargaville PA, Aiyappan A, De Paoli AG, Kuschel CA, Kamlin COF, et al. Minimally-invasive surfactant therapy in preterm infants on continuous positive airway pressure. Arch Dis Child Fetal Neonatal Ed. 2013;98:F122–6.
Göpel W, Kribs A, Ziegler A, Laux R, Hoehn T, et al. German Neonatal Network. Avoidance of mechanical ventilation by surfactant treatment of spontaneously breathing preterm infants (AMV): an open-label, randomized, controlled trial. Lancet. 2011;378:1627–34.
Bertini G, Coviello C, Gozzini E, Bianconi T, Bresci C, et al. Change of cerebral oxygenation during surfactant treatment in preterm infants:“LISA” versus “InSurE” procedures. Neuropediatrics. 2017;48:98–103.
Gallup JA, Ndakor SM, Pezzano C, Pinheiro JMB. Randomized trial of surfactant therapy via laryngeal mask airway versus brief tracheal intubation in neonates norn preterm. J Pediatr. 2023;254:17–24.
Pinheiro JM, Santana-Rivas Q, Pezzano C. Randomized trial of laryngeal mask airway versus endotracheal intubation for surfactant delivery. J Perinatol. 2016;36:196–201.
Barbosa RF, Simões E, Silva AC, Silva YP. A randomized controlled trial of the laryngeal mask airway for surfactant administration in neonates. J Pediatr (Rio J). 2017;93:343–50.
Amini E, Sheikh M, Shariat M, Dalili H, Azadi N, et al. Surfactant administration in preterm neonates using laryngeal mask airway: a randomized clinical trial. Acta Med Iran. 2019;57:348–54.
Attridge JT, Stewart C, Stukenborg GJ, Kattwinkel J. Administration of rescue surfactant by laryngeal mask airway: lessons from a pilot trial. Am J Perinatol. 2013;30:201–6.
Roberts KD, Brown R, Lampland AL, Leone TA, Rudser KD, et al. Laryngeal mask airway for surfactant administration in neonates: a randomized, controlled trial. J Pediatr. 2018;193:40–46.
Gharehbaghi M, Moghaddam YJ, Radfar R. Comparing the efficacy of surfactant administration by laryngeal mask airway and endotracheal intubation in neonatal respiratory distress syndrome. Crescent J Med Biol Sci. 2018;5:222–7.
Sadeghnia A, Tanhaei M, Mohammadizadeh M, Nemati M. A comparison of surfactant administration through i-gel and ET-tube in the treatment of respiratory distress syndrome in newborns weighing more than 2000 grams. Adv Biomed Res. 2014;3:160.
Kubicka Z, Zahr E, Rousseau T, Feldman HA, Fiascone J. Quality improvement to reduce chronic lung disease rates in very-low birth weight infants: high compliance with a respiratory care bundle in a small NICU. J Perinatol. 2018;38:285–92.
Tracy MB, Priyadarshi A, Goel D, Lowe K, Huvanandana J, et al. How do different brands of size 1 laryngeal mask airway compare with face mask ventilation in a dedicated laryngeal mask airway teaching manikin? Arch Dis Child Fetal Neonatal Ed. 2018;103:F271–6.
Wanous A, Brown R, Rudser K, Roberts K. Comparison of laryngeal mask airway and endotracheal tube placement in neonates. J Perinatol. 2023;44:239–43.
Foglia EE, Ades A, Napolitano N, Leffelman J, Nadkarni V, Nishisaki A. Factors associated with adverse events during tracheal intubation in the NICU. Neonatology. 2015;108:23–9.
Hatch LD, Grubb PH, Lea AS, Walsh WF, Markham MH, Whitney GM, et al. Endotracheal intubation in neonates: a prospective study of adverse safety events in 162 infants. J Pediatr. 2016;168:62–6.
Foglia EE, Ades A, Sawyer T, Glass KM, Singh N, Jung P, et al. Neonatal intubation practice and outcomes: an international registry study. Pediatrics. 2019;143:e20180902.
Hatch LD, Grubb PH, Lea AS, Walsh WF, Markham MH, Maynord PO, et al. Interventions to improve patient safety during intubation in the neonatal intensive care unit. Pediatrics. 2016;138:e20160069.
Roberts KD, Leone TA, Edwards WH, Rich WD, Finer NN. Premedication for nonemergent neonatal intubations: a randomized, controlled trial comparing atropine and fentanyl to atropine, fentanyl, and mivacurium. Pediatrics. 2006;118:1583–91.
Raimondi F, Yousef N, Migliaro F, Capasso L, De Luca D. Point-of-care lung ultrasound in neonatology: classification into descriptive and functional applications. Pediatr Res. 2021;90:524–53.
Acknowledgements
We would like to thank South Shore Hospital NICU respiratory therapists and nurses for their support with clinical protocol.
Author information
Authors and Affiliations
Contributions
ZK conceptualized and designed the study, designed the data collection instruments, coordinated and supervised data collection, collected data, drafted the initial manuscript, and reviewed and revised the manuscript. BA, TW, EZ, AD, DP, TR, and ML contributed to the study design, data collection, and reviewed and revised the manuscript. CR RRT-NPS contributed to study design, created data capture tool, contributed to data collection, and reviewed and revised the manuscript. HAF conceptualized and designed the study, carried out the initial and final statistical analyses, and critically reviewed the manuscript for important intellectual content. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
41372_2024_2099_MOESM1_ESM.doc
“Guideline for Surfactant administration by LMA”, “LMA for Surfactant Administration Algorithm”, “LMA/Surfactant capture tool”
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kubicka, Z., Zahr, E., Feldman, H.A. et al. Feasibility and safety of surfactant administration via laryngeal mask airway as first-line therapy for a select newborn population: results of a standardized clinical protocol. J Perinatol 45, 36–42 (2025). https://doi.org/10.1038/s41372-024-02099-8
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41372-024-02099-8