Abstract
Objective
To compare the efficacy of nebulized salbutamol in reducing respiratory distress in late preterm and term neonates with transient tachypnea of newborn (TTN).
Study design
Double-blind, placebo-controlled randomized trial.
Methods
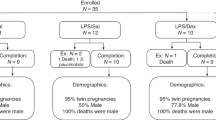
Neonates with TTN (n = 134) were allocated to nebulized salbutamol (n = 67) versus placebo (normal saline) (n = 67). The primary outcome was the duration of tachypnea (respiratory rate >60/min). Secondary outcomes were maximum Downes score and fraction of inspired oxygen (FiO2) after nebulization, duration of respiratory support, and adverse effects of salbutamol nebulization.
Results
Median duration of tachypnea was 12.5 (10–16) vs. 12 (10.4–14) hours in salbutamol and placebo groups, respectively; p = 0.489. Almost all neonates received positive-pressure respiratory support at delivery room and subsequently. Maximum Downe’s score, FiO2 requirement, and duration of respiratory support were similar. No adverse effect of salbutamol was documented.
Conclusion
There was no difference in the duration of tachypnea with nebulized salbutamol compared to placebo in late preterm and term neonates with TTN.
Clinical trial registration
Clinical trial registry of India, Registration no: CTRI/2023/05/052441, Registered prospectively on 10/05/2023, https://ctri.icmr.org.in/
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
References
Clark RH. The epidemiology of respiratory failure in neonates born at an estimated gestational age of 34 weeks or more. J Perinatol. 2005;25:251–7.
Edwards MO, Kotecha SJ, Kotecha S. Respiratory distress of the term newborn infant. Paediatr Respir Rev. 2013;14:29–36.
Hansen AK, Wisborg K, Uldbjerg N, Henriksen TB. Risk of respiratory morbidity in term infants delivered by elective caesarean section: cohort study. BMJ. 2008;336:85–7.
Hagen E, Chu A, Lew C. Transient tachypnea of the newborn. NeoReviews. 2017;18:e141.
Liem JJ, Huq SI, Ekuma O, Becker AB, Kozyrskyj AL. Transient tachypnea of the newborn may be an early clinical manifestation of wheezing symptoms. J Pediatr. 2007;151:29–33.
Tudehope DI, Smyth MH. Is “transient tachypnoea of the newborn” always a benign disease? Report of 6 babies requiring mechanical ventilation. Aust Paediatr J. 1979;15:160–5.
Jha K, Nassar GN, Makker K. Transient Tachypnea of the Newborn. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2024. https://www.ncbi.nlm.nih.gov/books/NBK537354/
Dehdashtian M, Aletayeb M, Malakian A, Aramesh MR, Malvandi H. Clinical course in infants diagnosed with transient tachypnea of newborn: A clinical trial assessing the role of conservative versus conventional management. J Chin Med Assoc. 2018;81:183–6.
Bruschettini M, Hassan KO, Romantsik O, Banzi R, Calevo MG, Moresco L. Interventions for the management of transient tachypnoea of the newborn - an overview of systematic reviews. Cochrane Database Syst Rev. 2022;2:CD013563.
Mutlu GM, Koch WJ, Factor P. Alveolar epithelial beta 2-adrenergic receptors: their role in regulation of alveolar active sodium transport. Am J Respir Crit Care Med. 2004;170:1270–5.
Barker PM, Olver RE. Invited review: clearance of lung liquid during the perinatal period. J Appl Physiol. 2002;93:1542–8.
Rawlings JS, Smith FR. Transient tachypnea of the newborn. An analysis of neonatal and obstetric risk factors. Am J Dis Child. 1984;138:869–71.
Downes JJ, Vidyasagar D, Boggs TR Jr, Morrow GM 3rd. Respiratory distress syndrome of newborn infants. I. New clinical scoring system (RDS score) with acid-base and blood-gas correlations. Clin Pediatr. 1970;9:325–31.
Bertrand P, Araníbar H, Castro E, Sánchez I. Efficacy of nebulized epinephrine versus salbutamol in hospitalized infants with bronchiolitis. Pediatr Pulmonol. 2001;31:284–8.
Kim MJ, Yoo JH, Jung JA, Byun SY. The effects of inhaled albuterol in transient tachypnea of the newborn. Allergy Asthma Immunol Res. 2014;6:126–30.
Armangil D, Yurdakök M, Korkmaz A, Yiğit S, Tekinalp G. Inhaled beta-2 agonist salbutamol for the treatment of transient tachypnea of the newborn. J Pediatr. 2011;159:398–403.e1.
Mohammadzadeh I, Akbarian-Rad Z, Heidari F, Zahedpasha Y, Haghshenas-Mojaveri M. The effect of inhaled salbutamol in transient of tachypnea of the newborn: a randomized clinical trial. Iran J Pediatr. 2017;27:e9633.
Mussavi M, Asadollahi K, Kayvan M, Sadeghvand S. Effects of nebulized albuterol in transient tachypnea of the newborn a clinical trial. Iran J Pediatr. 2017;27:e8211.
Babaei H, Dabiri S, Mohammadi Pirkashani L, Mohsenpour H. Effects of salbutamol on the treatment of transient tachypnea of the newborn. Iran J Neonatol. 2019:10. https://doi.org/10.22038/ijn.2018.31294.1430
Talaat AA, Abohashish MMA, Farid TM, Salah MM. Evaluation of inhaled beta-2 agonist in management of transient tachypnea of the newborn. Bull Natl Res Cent. 2020;44:12.
Basiri B, Sadeghi N, Sabzehei MK, Ashari FE. Effects of inhaled salbutamol on transient tachypnea of the newborn. Respir Care. 2022;67:433–9.
Malakian A, Dehdashtian M, Aramesh MR, Aletayeb MH, Heidari S. The effect of inhaled salbutamol on the outcomes of transient tachypnea of the newborn. J Chin Med Assoc. 2018;81:990–7.
Moresco L, Bruschettini M, Macchi M, Calevo MG. Salbutamol for transient tachypnea of the newborn. Cochrane Database Syst Rev. 2021;2:CD011878.
Pillow JJ, Hillman N, Moss TJ, Polglase G, Bold G, Beaumont C, et al. Bubble continuous positive airway pressure enhances lung volume and gas exchange in preterm lambs. Am J Respir Crit Care Med. 2007;176:63–9.
Acknowledgements
We wish to convey our sincere thanks to the neonatologists and NICU staff members at All India Institute of Medical Sciences, Rishikesh for participating in the study.
Author information
Authors and Affiliations
Contributions
Drs. Dhaka and Kumar recruited patients, collected and analyzed the data, and drafted the initial manuscript; Drs. Basu, Singh, and Priyadarshi supervised data collection and analysis of data and did critical revision and finalization of the manuscript; Drs. Chaurasia and Bhat contributed to the study design, data analysis and interpretation; and all authors approved the final manuscript as submitted.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. The study protocol was approved by the Institute’s Ethics Committee of AIIMS Rishikesh, Uttarakhand, India (Ref No: AIIMS/IEC/23/143, dated 28/04/2023).
Consent to participate
Written informed consent was obtained from the parents of each subject before enrolment.
Consent for publication
Patients signed informed consent regarding publishing their data and photographs.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dhaka, A., Kumar, S., Singh, P. et al. Nebulized salbutamol for the treatment of transient tachypnea of the newborn: a randomized controlled trial. J Perinatol 45, 1595–1600 (2025). https://doi.org/10.1038/s41372-024-02201-0
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41372-024-02201-0
This article is cited by
-
Effect of Nebulized Salbutamol on Lung Ultrasound Aeration Score in Transient Tachypnea of Newborn
Indian Journal of Pediatrics (2026)