Abstract
Objective
To determine postnatal changes in total serum bilirubin (TB), unbound bilirubin (UB), and bilirubin-binding affinity of albumin (Ka) among Japanese newborns.
Study design
In a retrospective study, we evaluated for serum TB, UB, and albumin (Alb) levels, and their calculated UB/TB ratios, and Ka in 786 neonates born ≥36 weeks’ gestation and analyzed to subcategories of three postnatal epochs: first, second, and ≥third weeks.
Result
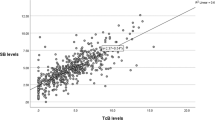
TB levels were significantly higher (p < 0.01) at age ≥three weeks, while UB levels were significantly higher (p < 0.01) in the first week. UB/TB ratios were significantly lower while calculated Ka values were significantly higher as age-in-weeks increased (p < 0.01).
Conclusion
Hyperbilirubinemia in the first and second weeks of age in neonates born ≥36 weeks’ gestation is associated with higher UB levels or UB/TB ratios with lower Ka and may potentially contribute to the risk of developing bilirubin neurotoxicity with aggravated variations in bilirubin, albumin and Ka.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
References
ODELL GB. The dissociation of bilirubin from albumin and its clinical implications. J Pediatr. 1959;55:268–79.
Wennberg RP, Ahlfors CE, Rasmussen LF. The pathochemistry of kernicterus. Early Hum Dev. 1979;3:353–72.
Lai NM, Gerard JP, Ngim CF, Kamar AA, Chen KH. The association between serum bilirubin and kernicterus spectrum disorder: A systematic review and meta-analysis. Neonatology. 2021;118:654–64.
Nakamura H, Takada S, Shimabuku R, Matsuo M, Matsuo T, Negishi H. Auditory nerve and brainstem responses in newborn infants with hyperbilirubinemia. Pediatrics. 1985;75:703–8.
Amin SB, Wang H, Laroia N, Orlando M. Unbound bilirubin and auditory neuropathy spectrum disorder in late preterm and term infants with severe jaundice. J Pediatr. 2016;173:84–89.
Amin SB, Saluja S, Saili A, Orlando M, Wang H, Laroia N, et al. Chronic auditory toxicity in late preterm and term infants with significant hyperbilirubinemia. Pediatrics. 2017;140:e20164009. https://doi.org/10.1542/peds.2016-4009.
Amin SB. bilirubin binding capacity in the preterm neonate. Clin Perinatol. 2016;43:241–57.
Katayama Y, Enomoto M, Kikuchi S, Takei A, Ikegami H, Minami H, et al. Transcutaneous bilirubin measurement during phototherapy in term neonates. Pediatr Int. 2017;59:686–90.
Morioka I. Hyperbilirubinemia in preterm infants in Japan: New treatment criteria. Pediatr Int. 2018;60:684–90.
Kemper AR, Newman TB, Slaughter JL, Maisels MJ, Watchko JF, Downs SM, et al. Clinical Practice Guideline Revision: Management of Hyperbilirubinemia in the Newborn Infant 35 or More Weeks of Gestation. Pediatrics. 2022;150:e2022058859. https://doi.org/10.1542/peds.2022-058859.
Nakamura H, Lee Y. Microdetermination of unbound bilirubin in icteric newborn sera: an enzymatic method employing peroxidase and glucose oxidase. Clin Chim Acta. 1977;79:411–7.
Jacobsen J, Wennberg RP. Determination of unbound bilirubin in the serum of newborns. Clin Chem. 1974;20:783.
Lee YK, Daito Y, Katayama Y, Minami H, Negishi H. The significance of measurement of serum unbound bilirubin concentrations in high-risk infants. Pediatr Int. 2009;51:795–9.
Amin SB, Wang H. Bilirubin albumin binding and unbound unconjugated hyperbilirubinemia in premature infants. J Pediatr. 2018;192:47–52.
Sato Y, Morioka I, Miwa A, Yokota T, Matsuo K, Koda T, et al. Is bilirubin/albumin ratio correlated with unbound bilirubin concentration? Pediatr Int. 2012;54:81–85.
Flaherman VJ, Maisels MJ, Academy of Breastfeeding Medicine. ABM Clinical Protocol #22: Guidelines for Management of Jaundice in the Breastfeeding Infant 35 Weeks or More of Gestation-Revised 2017. Breastfeed Med. 2017;12:250–7.
Maruo Y, Morioka Y, Fujito H, Nakahara S, Yanagi T, Matsui K, et al. Bilirubin uridine diphosphate-glucuronosyltransferase variation is a genetic basis of breast milk jaundice. J Pediatr. 2014;165:36–41.
Lamola AA, Bhutani VK, Du L, Castillo Cuadrado M, Chen L, Shen Z, et al. Neonatal bilirubin binding capacity discerns risk of neurological dysfunction. Pediatr Res. 2015;77:334–9.
Amin SB. Narrative review of bilirubin measurement and binding. Pediatr Med. 2021;4:33.
Meisel P, Biebler KE, Gens A, Jaehrig K. Albumin binding of photobilirubin II. Biochem J. 1983;213:25–29.
Stevenson DK, Fanaroff AA, Maisels MJ, Young BW, Wong RJ, Vreman HJ, et al. Prediction of hyperbilirubinemia in near-term and term infants. Pediatrics. 2001;108:31–39.
Bhutani VK, Stark AR, Lazzeroni LC, Poland R, Gourley GR, Kazmierczak S, et al. Initial Clinical Testing Evaluation and Risk Assessment for Universal Screening for Hyperbilirubinemia Study Group. Predischarge screening for severe neonatal hyperbilirubinemia identifies infants who need phototherapy. J Pediatr. 2013;162:477–82.
Bentz MG, Carmona N, Bhagwat MM, Thimmig LM, Saleh J, Eke U, et al. Beyond “Asian”: specific East and Southeast Asian races or ethnicities associated with jaundice readmission. Hosp Pediatr. 2018;8:269–73.
Acknowledgements
The authors sincerely appreciate Dr. Ronald J. Wong (Department of Pediatrics, Stanford University School of Medicine) for his critical review and editing of this manuscript.
Author information
Authors and Affiliations
Contributions
KN: Data curation, formal analysis, original draft preparation. YK: Conceptualization, writing-reviewing and editing, Supervision. YL: Conceptualization, reviewing and editing. All authors approved the final manuscript as submitted.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
This study was approved by the ethics committee of Takatsuki General Hospital (approval number: 2021–20) and was performed in accordance with the Declaration of Helsinki.
Consent to participate
The ethics committee of Takatsuki General Hospital approved this observational study with a waiver of informed parental consent.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nishizawa, K., Katayama, Y. & Lee, YK. Unbound bilirubin and bilirubin-albumin binding levels of Japanese neonates. J Perinatol 45, 965–970 (2025). https://doi.org/10.1038/s41372-025-02266-5
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41372-025-02266-5