Abstract
Objective
To evaluate the longitudinal changes of IL-1α, IL-1β, and IL-1Ra in extremely preterm infants and investigate the dynamic association with bronchopulmonary dysplasia (BPD).
Methods
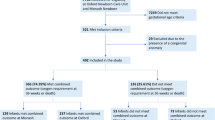
Plasma samples were collected from extremely preterm infants at postnatal day (PD) 7,28 and PMA 36 weeks. IL-1 cytokines concentrations were measured by Bio-Plex Pro (human cytokine panel). Univariate and multivariate logistic regression analysis were conducted to explore the association between the cytokines and BPD.
Results
BPD infants exhibited significantly higher concentrations of IL-1α (10.75 vs. 8.18 pg/ml, p = 0.026), IL-1β (2.00 vs. 1.50 pg/ml, p = 0.046), and IL-1Ra (878.50 vs. 262.40 pg/ml, p = 0.011) compared to non-BPD infants at PD 28. Higher IL-1α concentration (≥8.09 pg/ml) at PD 28 was independently associated with BPD development (OR: 8.272, 95% CI: 1.127–60.705, p = 0.038).
Conclusions
Increased IL-1α concentrations at PD 28 were independently associated with an increased risk of BPD.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Gilfillan M, Bhandari A, Bhandari V. Diagnosis and management of bronchopulmonary dysplasia. BMJ. 2021;375:n1974.
Stoll BJ, Hansen NI, Bell EF, Shankaran S, Laptook AR, Walsh MC, et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics. 2010;126:443–56.
Bell EF, Hintz SR, Hansen NI, Bann CM, Wyckoff MH, De Mauro SB, et al. Mortality, In-Hospital Morbidity, Care Practices, and 2-Year Outcomes for Extremely Preterm Infants in the US, 2013–2018. JAMA. 2022;327:248–63.
Savani RC. Modulators of inflammation in bronchopulmonary dysplasia. Semin Perinatol. 2018;42:459–70.
Álvarez-Fuente M, Moreno L, Mitchell JA, Reiss IK, Lopez P, Elorza D, et al. Preventing bronchopulmonary dysplasia: new tools for an old challenge. Pediatr Res. 2019;85:432–41.
Rudloff I, Cho SX, Bui CB, McLean C, Veldman A, Berger PJ, et al. Refining anti-inflammatory therapy strategies for bronchopulmonary dysplasia. J Cell Mol Med. 2017;21:1128–38.
Dinarello CA. Overview of the IL-1 family in innate inflammation and acquired immunity. Immunol Rev. 2018;281:8–27.
Garlanda C, Dinarello CA, Mantovani A. The interleukin-1 family: back to the future. Immunity. 2013;39:1003–18.
Sims JE, March CJ, Cosman D, Widmer MB, MacDonald HR, McMahan CJ, et al. cDNA expression cloning of the IL-1 receptor, a member of the immunoglobulin superfamily. Science. 1988;241:585–9.
Sims JE, Smith DE. The IL-1 family: regulators of immunity. Nat Rev Immunol. 2010;10:89–102.
Fritzsching B, Zhou-Suckow Z, Trojanek JB, Schubert SC, Schatterny J, Hirtz S, et al. Hypoxic epithelial necrosis triggers neutrophilic inflammation via IL-1 receptor signaling in cystic fibrosis lung disease. Am J Respir Crit Care Med. 2015;191:902–13.
Borthwick LA. The IL-1 cytokine family and its role in inflammation and fibrosis in the lung. Semin Immunopathol. 2016;38:517–34.
Omer M, Melo AM, Kelly L, Mac Dermott EJ, Leahy TR, Killeen O, et al. Emerging role of the NLRP3 inflammasome and interleukin-1β in Neonates. Neonatology. 2020;117:545–54.
Yoon BH, Romero R, Jun JK, Park KH, Park JD, Ghezzi F, et al. Amniotic fluid cytokines (interleukin-6, tumor necrosis factor-alpha, interleukin-1 beta, and interleukin-8) and the risk for the development of bronchopulmonary dysplasia. Am J Obstet Gynecol. 1997;177:825–30.
Kazzi SN, Romero R, McLaughlin K, Ager J, Janisse J. Serial changes in levels of IL-6 and IL-1beta in premature infants at risk for bronchopulmonary dysplasia. Pediatr Pulmonol. 2001;31:220–6.
Ambalavanan N, Carlo WA, D’Angio CT, McDonald SA, Das A, Schendel D, et al. Cytokines associated with bronchopulmonary dysplasia or death in extremely low birth weight infants. Pediatrics. 2009;123:1132–41.
Mao X, Qiu J, Zhao L, Xu J, Yin J, Yang Y, et al. Vitamin D and IL-10 deficiency in preterm neonates with bronchopulmonary dysplasia. Front Pediatr. 2018;6:246.
Zhang Z, Wu W, Hou L, Jiang J, Wan W, Li Z. Cytokines and exhaled nitric oxide are risk factors in preterm infants for bronchopulmonary dysplasia. Biomed Res Int. 2021;2021:6648208.
Butler B, De Dios R, Nguyen L, McKenna S, Ghosh S, Wright CJ. Developmentally regulated innate immune NFκB signaling mediates IL-1α expression in the perinatal murine lung. Front Immunol. 2019;10:1555.
Eldredge LC, Creasy RS, Presnell S, Debley JS, Juul SE, Mayock DE, et al. Infants with evolving bronchopulmonary dysplasia demonstrate monocyte-specific expression of IL-1 in tracheal aspirates. Am J Physiol Lung Cell Mol Physiol. 2019;317:L49–l56.
Nold MF, Mangan NE, Rudloff I, Cho SX, Shariatian N, Samarasinghe TD, et al. Interleukin-1 receptor antagonist prevents murine bronchopulmonary dysplasia induced by perinatal inflammation and hyperoxia. Proc Natl Acad Sci USA. 2013;110:14384–9.
Gupta GK, Cole CH, Abbasi S, Demissie S, Njinimbam C, Nielsen HC, et al. Effects of early inhaled beclomethasone therapy on tracheal aspirate inflammatory mediators IL-8 and IL-1ra in ventilated preterm infants at risk for bronchopulmonary dysplasia. Pediatr Pulmonol. 2000;30:275–81.
Kakkera DK, Siddiq MM, Parton LA. Interleukin-1 balance in the lungs of preterm infants who develop bronchopulmonary dysplasia. Biol Neonate. 2005;87:82–90.
Jensen EA, Dysart K, Gantz MG, McDonald S, Bamat NA, Keszler M, et al. The diagnosis of bronchopulmonary dysplasia in very preterm infants. an evidence-based approach. Am J Respir Crit Care Med. 2019;200:751–9.
Davis P, Turner-Gomes S, Cunningham K, Way C, Roberts R, Schmidt B. Precision and accuracy of clinical and radiological signs in premature infants at risk of patent ductus arteriosus. Arch Pediatr Adolesc Med. 1995;149:1136–41.
Dani C, Bertini G, Reali MF, Murru P, Fabris C, Vangi V, et al. Prophylaxis of patent ductus arteriosus with ibuprofen in preterm infants. Acta Paediatr. 2000;89:1369–74.
Subspecialty Group of Neonatology, the Society of Pediatric, Chinese Medical Association; Professional Committee of Infectious Diseases, Neonatology Society, Chinese Medical Doctor Association. Expert consensus on the diagnosis and management of neonatal sepsis (version 2019). Zhonghua Er Ke Za Zhi. 2019;57:252–7.
Willet KE, Kramer BW, Kallapur SG, Ikegami M, Newnham JP, Moss TJ, et al. Intra-amniotic injection of IL-1 induces inflammation and maturation in fetal sheep lung. Am J Physiol Lung Cell Mol Physiol. 2002;282:L411–420.
Sosenko IR, Kallapur SG, Nitsos I, Moss TJ, Newnham JP, Ikegami M, et al. IL-1 alpha causes lung inflammation and maturation by direct effects on preterm fetal lamb lungs. Pediatr Res. 2006;60:294–8.
Rider P, Carmi Y, Voronov E, Apte RN. Interleukin-1α. Semin Immunol. 2013;25:430–8.
Osei ET, Noordhoek JA, Hackett TL, Spanjer AI, Postma DS, Timens W, et al. Interleukin-1α drives the dysfunctional cross-talk of the airway epithelium and lung fibroblasts in COPD. Eur Respir J. 2016;48:359–69.
Aden N, Nuttall A, Shiwen X, de Winter P, Leask A, Black CM, et al. Epithelial cells promote fibroblast activation via IL-1alpha in systemic sclerosis. J Invest Dermatol. 2010;130:2191–2200.
Piper SC, Ferguson J, Kay L, Parker LC, Sabroe I, Sleeman MA, et al. The role of interleukin-1 and interleukin-18 in pro-inflammatory and anti-viral responses to rhinovirus in primary bronchial epithelial cells. PLoS One. 2013;8:e63365.
Montgomery ST, Dittrich AS, Garratt LW, Turkovic L, Frey DL, Stick SM, et al. Interleukin-1 is associated with inflammation and structural lung disease in young children with cystic fibrosis. J Cyst Fibros. 2018;17:715–22.
Liao J, Kapadia VS, Brown LS, Cheong N, Longoria C, Mija D, et al. The NLRP3 inflammasome is critically involved in the development of bronchopulmonary dysplasia. Nat Commun. 2015;6:8977.
Green EA, Metz D, Galinsky R, Atkinson R, Skuza EM, Clark M, et al. Anakinra Pilot - a clinical trial to demonstrate safety, feasibility and pharmacokinetics of interleukin 1 receptor antagonist in preterm infants. Front Immunol. 2022;13:1022104.
Solomonidi N, Vlachoyiannopoulos PG, Pappa M, Liantinioti G, Ktena S, Theotikos E, et al. A randomized clinical trial of bermekimab treatment for clinical improvement of systemic sclerosis. iScience. 2023;26:107670.
Markota Čagalj A, Marinović B, Bukvić Mokos Z. New and emerging targeted therapies for hidradenitis suppurativa. Int J Mol Sci. 2022;23:3753.
Tao X, Mo L, Zeng L. Hyperoxia induced bronchopulmonary dysplasia-like inflammation via miR34a-TNIP2-IL-1β pathway. Front Pediatr. 2022;10:805860.
Köksal N, Kayik B, Çetinkaya M, Özkan H, Budak F, Kiliç Ş, et al. Value of serum and bronchoalveolar fluid lavage pro- and anti-inflammatory cytokine levels for predicting bronchopulmonary dysplasia in premature infants. Eur Cytokine Netw. 2012;23:29–35.
Rocha G, Proença E, Guedes A, Carvalho C, Areias A, Ramos JP, et al. Cord blood levels of IL-6, IL-8 and IL-10 may be early predictors of bronchopulmonary dysplasia in preterm newborns small for gestational age. Dis Markers. 2012;33:51–60.
Doyle LW. Postnatal corticosteroids to prevent or treat bronchopulmonary dysplasia. Neonatology. 2021;118:244–51.
Rindfleisch MS, Hasday JD, Taciak V, Broderick K, Viscardi RM. Potential role of interleukin-1 in the development of bronchopulmonary dysplasia. J Interferon Cytokine Res. 1996;16:365–73.
Acknowledgements
We gratefully acknowledge Zilu Huang and Yijun Liu for their assistance in acquiring the blood samples.
Funding
This study is supported by National Natural Science Foundation of China (82101803 & 82371707 & 32100082), Sanming Project of Medicine in Shenzhen (SZSM202211001), and Shenzhen Key Laboratory of Maternal and Child Health and Diseases (ZDSYS20230626091559006).
Author information
Authors and Affiliations
Contributions
XYC, CZY, and QLL conceived the study and designed the experiments. QLL, XYC, CHL, and YBR carried out the clinical data collection. DSH, XW, and LLY collected the clinical samples. QLL, XYC, and YRC performed data analysis. QLL and XYC wrote the manuscript. XYC and CZY reviewed and revised the manuscript. All authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
The study was approved by the Shenzhen Maternity & Child Healthcare Hospital Institutional Ethical Committee [SFYLS (2021) 019].
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, Q., Li, C., Rao, Y. et al. Dynamic association between plasma interleukin-1 family concentrations and bronchopulmonary dysplasia in extremely premature infants. J Perinatol (2025). https://doi.org/10.1038/s41372-025-02275-4
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41372-025-02275-4