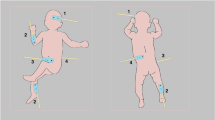
Abstract
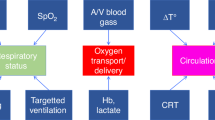
Organ perfusion and regional tissue oxygen saturation (rSO2) can be measured non-invasively using near-infrared spectroscopy (NIRS). While cerebral NIRS monitoring in neonates has been widely used, the adoption of renal NIRS is still evolving. This narrative review explores the application of renal NIRS in neonates and proposes an algorithm for integrating renal and cerebral NIRS in the neonatal intensive care unit. Decreased renal regional oxygenation (RrSO2) suggests decreased renal O2 delivery/perfusion or increased O2 consumption, warranting evaluation for acute kidney injury, anemia, hemodynamically significant patent ductus arteriosus, or hypotension. Increased RrSO2 indicates increased renal O2 delivery/perfusion or decreased O2 consumption, necessitating assessment for hyperoxia or established kidney injury. Combining cerebral and renal NIRS provides a comprehensive evaluation, allowing for the detection of early clinical changes. This integrated monitoring approach holds promise for improving neonatal outcomes. However, further large-scale studies are needed to establish normal ranges and guide therapeutic interventions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Sood BG, McLaughlin K, Cortez J. Near-infrared spectroscopy: applications in neonates. Semin Fetal Neonatal Med. 2015;20:164–72.
Pellicer A, Bravo. MdC. Near-infrared spectroscopy: a methodology-focused review. Semin Fetal Neonatal Med. 2011;16:42–49.
Mintzer JP, Moore JE. Regional tissue oxygenation monitoring in the neonatal intensive care unit: evidence for clinical strategies and future directions. Pediatr Res. 2019;86:296–304.
Altit G, Bhombal S, Chock VY. End-organ saturations correlate with aortic blood flow estimates by echocardiography in the extremely premature newborn—an observational cohort study. BMC Pediatr. 2021;21:312–20.
Bailey S, Hendricks-Munoz K, Mally P. Cerebral, renal, and splanchnic tissue oxygen saturation values in healthy term newborns. Am J Perinatol. 2014;31:339–44.
Bernal NP, Hoffman GM, Ghanayem NS, Arca MJ. Cerebral and somatic near-infrared spectroscopy in normal newborns. J Pediatr Surg. 2010;45:1306–10.
Harer MW, Condit PE, Chuck JE, Lasarev MR, Chock VY. Renal oxygenation measured by near-infrared spectroscopy in preterm neonates in the first week. Pediatr Res. 2022;92:1744–8.
Hoffman SB, Magder LS, Viscardi RM. Renal versus cerebral saturation trajectories: the perinatal transition in preterm neonates. Pediatr Res. 2022;92:1437–42.
Marin T, Williams BL. Renal oxygenation measured by near-infrared spectroscopy in neonates. Adv Neonatal Care. 2021;21:256–66.
McNeill S, Gatenby JC, McElroy S, Engelhardt B. Normal cerebral, renal and abdominal regional oxygen saturations using near-infrared spectroscopy in preterm infants. J Perinatol. 2011;31:51–57.
Montaldo P, De Leonibus C, Giordano L, De Vivo M, Giliberti P. Cerebral, renal and mesenteric regional oxygen saturation of term infants during transition. J Pediatr Surg. 2015;50:1273–7.
Harer MW, Gadek L, Rothwell AC, Richard L, Starr MC, Adegboro CO. Correlation of renal tissue oxygenation to venous, arterial, and capillary blood gas oxygen saturation in preterm neonates. Am J Perinatol. 2023;41:e1228–e1234.
Kazmi SH, Verma S, Bailey SM, Mally P, Desai P. Changes in regional tissue oxygen saturation values during the first week of life in stable preterm infants. J Perinat Med. 2024;52:445–51.
Kooi EMW, Mintzer JP, Rhee CJ, Ergenekon E, Schwarz CE, Pichler G, et al. Neonatal somatic oxygenation and perfusion assessment using near-infrared spectroscopy. Pediatr Res. 2024;96:1180–94.
Jeon GW. Clinical application of near-infrared spectroscopy in neonates. Neonatal Med. 2019;26:121–7.
Pavlek LR, Mueller C, Jebbia MR, Kielt MJ, Fathi O. Near-infrared spectroscopy in extremely preterm infants. Front Pediatr. 2021;8:624113–9.
Agudelo-Pérez S, Troncoso G, Botero-Rosas D, Muñoz C, Rodríguez A, Gómez AV, et al. Renal Regional Oxygen Saturation and Acute Kidney Injury in Neonates with Perinatal Asphyxia. Am J Perinatol. 2025;42:379–86.
Chock VY, Frymoyer A, Yeh CG, Van Meurs KP. Renal saturation and acute kidney injury in neonates with hypoxic ischemic encephalopathy undergoing therapeutic hypothermia. J Pediatr. 2018;200:232–239.e231.
Rumpel JA, Spray BJ, Frymoyer A, Rogers S, Cho S-H, Ranabothu S, et al. Renal oximetry for early acute kidney injury detection in neonates with hypoxic ischemic encephalopathy receiving therapeutic hypothermia. Pediatr Nephrol. 2023;38:2839–49.
Wu TW, Tamrazi B, Soleymani S, Seri I, Noori S. Hemodynamic changes during rewarming phase of whole-body hypothermia therapy in neonates with hypoxic-ischemic encephalopathy. J Pediatr. 2018;197:68–74.e62.
Bonsante F, Ramful D, Binquet C, Samperiz S, Daniel S, Gouyon JB, et al. Low renal oxygen saturation at near-infrared spectroscopy on the first day of life is associated with developing acute kidney injury in very preterm infants. Neonatology. 2019;115:198–204.
Harer MW, Adegboro CO, Richard LJ, McAdams RM. Non-invasive continuous renal tissue oxygenation monitoring to identify preterm neonates at risk for acute kidney injury. Pediatr Nephrol. 2021;36:1617–25.
Bhatt M, Petrova A, Mehta R. Does treatment of patent ductus arteriosus with cyclooxygenase inhibitors affect neonatal regional tissue oxygenation? Pediatr Cardiol. 2012;33:1307–14.
Petrova A, Bhatt M, Mehta R. Regional tissue oxygenation in preterm born infants in association with echocardiographically significant patent ductus arteriosus. J Perinatol. 2011;31:460–4.
Underwood MA, Milstein JM, Sherman MP. Near-infrared spectroscopy as a screening tool for patent ductus arteriosus in extremely low birth weight infants. Neonatology. 2007;91:134–9.
Arman D, Sancak S, Gürsoy T, Topcuoğlu S, Karatekin G, Ovalı F. The association between NIRS and Doppler ultrasonography in preterm infants with patent ductus arteriosus. J Matern-Fetal Neonatal Med. 2019;33:1245–52.
Arriaga-Redondo M, Rodríguez-Sánchez de la Blanca A, Zunzunegui JL, Ballesteros-Tejerizo F, Rodríguez-Ogando A, González-Navarro P, et al. Impact of catheterized ductal closure on renal and cerebral oximetry in premature neonates. Eur J Pediatr. 2024;183:2753–61.
Chock VY, Rose LA, Mante JV, Punn R. Near-infrared spectroscopy for detection of a significant patent ductus arteriosus. Pediatr Res. 2016;80:675–80.
Guzoglu N, Sari FN, Ozdemir R, Oguz SS, Uras N, Altug N, et al. Renal and mesenteric tissue oxygenation in preterm infants treated with oral ibuprofen. J Matern-Fetal Neonatal Med. 2014;27:197–203.
Navikienė J, Liubšys A, Viršilas E, Žvirblis T, Jankauskienė A. Impact of medical treatment of hemodynamically significant patent ductus arteriosus on cerebral and renal tissue oxygenation measured by near-infrared spectroscopy in very low-birth-weight infants. Medicina (Kaunas). 2022;58:475–83.
Navikiene J, Virsilas E, Vankeviciene R, Liubsys A, Jankauskiene A. Brain and renal oxygenation measured by NIRS related to patent ductus arteriosus in preterm infants: a prospective observational study. BMC Pediatr. 2021;21:559–65.
Rose LA, Frymoyer A, Bhombal S, Chock VY. Renal oxygen saturations and acute kidney injury in the preterm infant with patent ductus arteriosus. Am J Perinatol. 2023;41:e2606–e2612.
van der Laan ME, Roofthooft MTR, Fries MWA, Berger RMF, Schat TE, van Zoonen AGJF, et al. A hemodynamically significant patent ductus arteriosus does not affect cerebral or renal tissue oxygenation in preterm infants. Neonatology. 2016;110:141–7.
Dani C, Pratesi S, Fontanelli G, Barp J, Bertini G. Blood transfusions increase cerebral, splanchnic, and renal oxygenation in anemic preterm infants. Transfusion. 2010;50:1220–6.
Mintzer JP, Parvez B, Chelala M, Alpan G, LaGamma EF. Monitoring regional tissue oxygen extraction in neonates <1250 g helps identify transfusion thresholds independent of hematocrit. J Neonatal Perinat Med. 2014;7:89–100.
Mintzer JP, Parvez B, La Gamma EF. Regional tissue oxygen extraction and severity of anemia in very low birth weight neonates: a pilot NIRS analysis. Am J Perinatol. 2018;35:1411–8.
Seidel D, Blaser A, Gebauer C, Pulzer F, Thome U, Knupfer M. Changes in regional tissue oxygenation saturation and desaturations after red blood cell transfusion in preterm infants. J Perinatol. 2013;33:282–7.
Algra SO, Schouten ANJ, van Oeveren W, van der Tweel I, Schoof PH, Jansen NJG, et al. Low-flow antegrade cerebral perfusion attenuates early renal and intestinal injury during neonatal aortic arch reconstruction. J Thorac Cardiovascular Surg. 2012;144:1323–1328.e1322.
Altit G, Bhombal S, Tacy TA, Chock VY. End-organ saturation differences in early neonatal transition for left- versus right-sided congenital heart disease. Neonatology. 2018;114:53–61.
Berens RJ, Stuth EA, Robertson FA, Jaquiss RD, Hoffman GM, Troshynski TJ, et al. Near infrared spectroscopy monitoring during pediatric aortic coarctation repair. Paediatr Anaesth. 2006;16:777–81.
Condit PE, Gorski DP, Lasarev MR, Al-Subu AM, Harer MW. Decreased intraoperative renal tissue oxygenation after cardiopulmonary bypass predicts cardiac surgery-associated acute kidney injury in neonates. Children 2024;11:315–21.
Hoffman GM, Ghanayem NS, Scott JP, Tweddell JS, Mitchell ME, Mussatto KA. Postoperative cerebral and somatic near-infrared spectroscopy saturations and outcome in hypoplastic left heart syndrome. Ann Thorac Surg. 2017;103:1527–35.
Jung Kim H, Yeon Park J, Man Seo D, Jin Yun T, Park J-J, Gwak M. Acute kidney injury and renal regional oxygen saturation during aortic arch reconstruction in infants. J Cardiothorac Vasc Anesthesia. 2013;27:1153–7.
Sahni PV, Krishnamurthy G, Sahni R. Noninvasive monitoring to demonstrate postoperative differences in regional hemodynamics in newborn infants with d-transposition of the great arteries and hypoplastic left heart syndrome. World J Pediatr Congenit Heart Surg. 2022;14:194–200.
Uebing A, Furck AK, Hansen JH, Nufer E, Scheewe J, Dutschke P, et al. Perioperative cerebral and somatic oxygenation in neonates with hypoplastic left heart syndrome or transposition of the great arteries. J Thorac Cardiovasc Surg. 2011;142:523–30.
Beck J, Loron G, Masson C, Poli-Merol M-L, Guyot E, Guillot C, et al. Monitoring cerebral and renal oxygenation status during neonatal digestive surgeries using near infrared spectroscopy. Front Pediatr. 2017;5:140–7.
Conforti A, Giliberti P, Landolfo F, Valfre L, Columbo C, Mondi V, et al. Effects of ventilation modalities on near-infrared spectroscopy in surgically corrected CDH infants. J Pediatr Surg. 2016;51:349–53.
Conforti A, Giliberti P, Mondi V, Valfré L, Sgro S, Picardo S, et al. Near infrared spectroscopy: experience on esophageal atresia infants. J Pediatr Surg. 2014;49:1064–8.
Koch HW, Hansen TG, Kurth D. Perioperative use of cerebral and renal near‐infrared spectroscopy in neonates: a 24‐h observational study. Pediatr Anesthesia. 2015;26:190–8.
Lau PE, Cruz S, Garcia-Prats J, Cuevas M, Rhee C, Cass DL, et al. Use of renal near-infrared spectroscopy measurements in congenital diaphragmatic hernia patients on ECMO. J Pediatr Surg. 2017;52:689–92.
Miyake Y, Seo S, Kataoka K, Ochi T, Miyano G, Koga H, et al. Significant neonatal intraoperative cerebral and renal oxygen desaturation identified with near-infrared spectroscopy. Pediatr Surg Int. 2022;38:737–42.
Arslan U, Kavrut Ozturk N, Kavakli AS, Dagdelen HO. Comparison of the effects of anaesthesia methods used in caesarean delivery on neonatal cerebral and renal oxygenation: a randomised controlled trial. J Clin Med. 2024;13:873–82.
Harer MW, Rothwell AC, Richard LJ, Adegboro CO, McAdams RM. Renal tissue oxygenation after caffeine administration in preterm neonates. Pediatr Res. 2021;90:1171–6.
Montaldo P, Puzone S, Caredda E, Pugliese U, Inserra E, Cirillo G, et al. Impact of intrauterine growth restriction on cerebral and renal oxygenation and perfusion during the first 3 days after birth. Sci Rep. 2022;12:5067–75.
Richter AE, Schat TE, Van Braeckel KN, Scherjon SA, Bos AF, Kooi EM. The Effect of Maternal Antihypertensive Drugs on the Cerebral, Renal and Splanchnic Tissue Oxygen Extraction of Preterm Neonates. Neonatology. 2016;110:163–71.
Terstappen F, Paauw ND, Alderliesten T, Joles JA, Vijlbrief DC, Lely AT, et al. Elevated renal tissue oxygenation in premature fetal growth restricted neonates: An observational study. Plos One 2018;13:e0204268–78.
van der Laan ME, Schat TE, Olthuis AJ, Boezen HM, Bos AF, Kooi EMW. The association between multisite near-infrared spectroscopy and routine hemodynamic measurements in relation to short-term outcome in preterms with clinical sepsis. Neonatology. 2015;108:297–304.
Burns DA, Ciurczak EW. Handbook of near-infrared analysis 2007.
Germon TJ, Evans PD, Barnett NJ, Wall P, Manara AR, Nelson RJ. Cerebral near infrared spectroscopy: emitter-detector separation must be increased. Br J Anaesth. 1999;82:831–7.
Strangman G, Boas DA, Sutton JP. Non-invasive neuroimaging using near-infrared light. Biol Psychiatry. 2002;52:679–93.
Almajidy RK, Mankodiya K, Abtahi M, Hofmann UG. A newcomer’s guide to functional near infrared spectroscopy experiments. IEEE Rev Biomed Eng. 2020;13:292–308.
Grometto A, Pizzo B, Strozzi MC, Gazzolo F, Gazzolo D. Cerebral NIRS patterns in late preterm and very preterm infants becoming late preterm. J Matern-Fetal Neonatal Med. 2017;32:1124–9.
Watzman HM, Kurth CD, Montenegro LM, Rome J, Steven JM, Nicolson SC. Arterial and venous contributions to near-infrared cerebral oximetry. Anesthesiology. 2000;93:947–53.
Garvey AA, Dempsey EM. Applications of near infrared spectroscopy in the neonate. Curr Opin Pediatr. 2018;30:209–15.
Vanderhaegen J, Naulaers G, Vanhole C, De Smet D, Van Huffel S, Vanhaesebrouck S, et al. The effect of changes in tPCO2 on the fractional tissue oxygen extraction – as measured by near-infrared spectroscopy – in neonates during the first days of life. Eur J Paediatr Neurol. 2009;13:128–34.
Naulaers G, Meyns B, Miserez M, Leunens V, Van Huffel S, Casaer P, et al. Use of tissue oxygenation index and fractional tissue oxygen extraction as non-invasive parameters for cerebral oxygenation. Neonatology. 2007;92:120–6.
Toet MC, Lemmers PMA, van Schelven LJ, van Bel F. Cerebral oxygenation and electrical activity after birth asphyxia: their relation to outcome. Pediatrics. 2006;117:333–9.
Balegar V KK, Low GKK, Nanan RKH. Regional tissue oxygenation and conventional indicators of red blood cell transfusion in anaemic preterm infants. EClinicalMedicine. 2022;46:101365–74.
Farag MM, Ghazal HAELR, Ibrahim A, Hammad B. Near-infrared spectroscopy measured cerebral oxygenation in full-term infants during transition: an observational study. Egypt Pediatric Association Gaz. 2022;70:53–62.
Lemmers PMA, Toet M, van Schelven LJ, van Bel F. Cerebral oxygenation and cerebral oxygen extraction in the preterm infant: the impact of respiratory distress syndrome. Exp Brain Res. 2006;173:458–67.
Dix LML, van Bel F, Lemmers PMA. Monitoring cerebral oxygenation in neonates: an update. Front Pediatr. 2017;5:46–54.
van Bel F, Lemmers P, Naulaers G. Monitoring neonatal regional cerebral oxygen saturation in clinical practice: value and pitfalls. Neonatology. 2008;94:237–44.
Schneider A, Minnich B, Hofstätter E, Weisser C, Hattinger‐Jürgenssen E, Wald M. Comparison of four near‐infrared spectroscopy devices shows that they are only suitable for monitoring cerebral oxygenation trends in preterm infants. Acta Paediatrica. 2014;103:934–8.
Dix LM, van Bel F, Baerts W, Lemmers PM. Comparing near-infrared spectroscopy devices and their sensors for monitoring regional cerebral oxygen saturation in the neonate. Pediatr Res. 2013;74:557–63.
Harer MW, Chock VY. Renal tissue oxygenation monitoring-an opportunity to improve kidney outcomes in the vulnerable neonatal population. Front Pediatr. 2020;8:241.
Greisen G. Cerebral blood flow and oxygenation in infants after birth asphyxia. Clinically useful information? Early Hum Dev. 2014;90:703–5.
Szakmar E, Smith J, Yang E, Volpe JJ, Inder T, El-Dib M. Association between cerebral oxygen saturation and brain injury in neonates receiving therapeutic hypothermia for neonatal encephalopathy. J Perinatol. 2021;41:269–77.
Meena J, Kumar J, Kocharlakota JP, Gupta H, Mittal P, Kumar A, et al. Acute kidney injury in neonates: a meta-analysis. Pediatrics. 2024;154:e2023065182.
Condit PE, Chuck JE, Lasarev MR, Chock VY, Harer MW. Renal tissue oxygenation and development of AKI in preterm neonates born < 32 weeks’ gestational age in the first week of age. J Perinatol. 2024;44:434–8.
Iacobelli S, Bonsante F, Ferdinus C, Labenne M, Gouyon JB. Factors affecting postnatal changes in serum creatinine in preterm infants with gestational age <32 weeks. J Perinatol. 2008;29:232–6.
Shimada S, Kasai T, Konishi M, Fujiwara T. Effects of patent ductus arteriosus on left ventricular output and organ blood flows in preterm infants with respiratory distress syndrome treated with surfactant. J Pediatr. 1994;125:270–7.
Alderliesten T, Lemmers PM, van Haastert IC, de Vries LS, Bonestroo HJ, Baerts W, et al. Hypotension in preterm neonates: low blood pressure alone does not affect neurodevelopmental outcome. J Pediatr. 2014;164:986–91.
Pellicer A, Greisen G, Benders M, Claris O, Dempsey E, Fumagalli M, et al. The SafeBoosC phase II randomised clinical trial: a treatment guideline for targeted near-infrared-derived cerebral tissue oxygenation versus standard treatment in extremely preterm infants. Neonatology. 2013;104:171–8.
Li J, Zhang G, Holtby H, Bissonnette B, Wang G, Redington AN, et al. Carbon dioxide—a complex gas in a complex circulation: Its effects on systemic hemodynamics and oxygen transport, cerebral, and splanchnic circulation in neonates after the Norwood procedure. J Thorac Cardiovascular Surg. 2008;136:1207–14.
Jobes DR, Nicolson SC, Steven JM, Miller M, Jacobs ML, Norwood WI. Carbon dioxide prevents pulmonary overcirculation in hypoplastic left heart syndrome. Ann Thorac Surg. 1992;54:150–1.
Ramamoorthy C, Tabbutt S, Dean Kurth C, Steven JamesM, Montenegro LisaM, Durning S, et al. Effects of inspired hypoxic and hypercapnic gas mixtures on cerebral oxygen saturation in neonates with univentricular heart defects. Anesthesiology. 2002;96:283–8.
Hoffman GM, Scott JP, Stuth EA. Effects of arterial carbon dioxide tension on cerebral and somatic regional tissue oxygenation and blood flow in neonates after the norwood procedure with deep hypothermic cardiopulmonary bypass. Front Pediatr. 2022;10:762739–49.
Hanson SJ, Berens RJ, Havens PL, Kim MK, Hoffman GM. Effect of volume resuscitation on regional perfusion in dehydrated pediatric patients as measured by two-site near-infrared spectroscopy. Pediatr Emerg Care. 2009;25:150–3.
Ortmann LA, Fontenot EE, Seib PM, Eble BK, Brown R, Bhutta AT. Use of near-infrared spectroscopy for estimation of renal oxygenation in children with heart disease. Pediatr Cardiol. 2011;32:748–53.
Aviles-Otero N, Kumar R, Khalsa DD, Green G, Carmody JB. Caffeine exposure and acute kidney injury in premature infants with necrotizing enterocolitis and spontaneous intestinal perforation. Pediatr Nephrol. 2018;34:729–36.
Harer MW, Askenazi DJ, Boohaker LJ, Carmody JB, Griffin RL, Guillet R, et al. Association between early caffeine citrate administration and risk of acute kidney injury in preterm neonates. JAMA Pediatr. 2018;172–80.
Carmody JB, Harer MW, Denotti AR, Swanson JR, Charlton JR. Caffeine exposure and risk of acute kidney injury in a retrospective cohort of very low birth weight neonates. J Pediatr. 2016;172:63–68.e61.
Petrova A, Mehta R. Near-infrared spectroscopy in the detection of regional tissue oxygenation during hypoxic events in preterm infants undergoing critical care. Pediatr Crit Care Med. 2006;7:449–54.
El-Dib M, Soul JS. Monitoring and management of brain hemodynamics and oxygenation. Neonatal Neurology. Handbook of Clinical Neurology2019. p. 295-314.
Author information
Authors and Affiliations
Contributions
DR designed the study, drafted the initial manuscript, and critically reviewed and revised the manuscript. HC, and EA, critically reviewed and revised the manuscript. MED conceptualized and designed the study, and critically reviewed and revised the manuscript for important intellectual content. All authors approved the final manuscript as submitted and agreed to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Ethical review and approval were waived for this study as this review is based on the analysis of data from literature.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rallis, D., Christou, H., Abdulhayoglu, E. et al. A narrative review of the clinical applications of renal NIRS and integration with cerebral NIRS in the NICU. J Perinatol 45, 1655–1663 (2025). https://doi.org/10.1038/s41372-025-02303-3
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41372-025-02303-3
This article is cited by
-
Stealing nephrons—a review on how patent ductus arteriosus physiology impacts neonatal kidney health
Journal of Perinatology (2025)