Abstract
Objectives
Bronchopulmonary dysplasia (BPD) is associated with poor cognition. The association between BPD severity and neurodevelopmental disorders (NDD) has not been explored. We hypothesized increasing BPD severity is associated with increased risk of NDD.
Study design
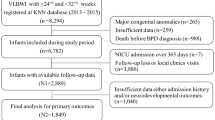
We included infants born <32 weeks’ gestation and birth weight <1500 g from 2015 to 2020 and excluded patients lost to follow-up or deceased before three years old. 650 patients were analyzed. BPD severity was defined as respiratory support at 36 weeks postmenstrual age. Risk factors of BPD were assessed. The primary outcome was composite NDD, including autism, attention deficit hyperactivity disorder, cerebral palsy, and/or learning disorders. Multiple logistic regression analyzed the association between BPD severity and outcomes, accounting for confounders.
Results
Increased BPD severity showed increased risk of NDD (p < 0.01). In a reduced model, BPD severity remained significant (p = 0.037). Tracheostomy was a risk factor for NDD (p = 0.004).
Conclusions
BPD severity should prompt suspicion for future NDD.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
The dataset utilized in this study is available from the corresponding author on reasonable request.
References
Ismail FY, Shapiro BK. What are neurodevelopmental disorders? Curr Opin Neurol. 2019;32:611–6. https://doi.org/10.1097/WCO.0000000000000710.
Reid SM, Meehan EM, Arnup SJ, Reddihough DS. Intellectual disability in cerebral palsy: a population-based retrospective study. Dev Med Child Neurol. 2018;60:687–94. https://doi.org/10.1111/dmcn.13773.
Zhang JY, Oskoui M, Shevell M. A population-based study of communication impairment in cerebral palsy. J Child Neurol. 2015;30:277–84. https://doi.org/10.1177/0883073814538497.
Korrel H, Mueller KL, Silk T, Anderson V, Sciberras E. Research review: language problems in children with attention-deficit hyperactivity disorder - a systematic meta-analytic review. J Child Psychol Psychiatry. 2017;58:640–54. https://doi.org/10.1111/jcpp.12688.
Avni E, Ben-Itzchak E, Zachor DA. The presence of comorbid ADHD and anxiety symptoms in autism spectrum disorder: clinical presentation and predictors. Front Psychiatry. 2018;20:9–717. https://doi.org/10.3389/fpsyt.2018.00717.
Houwen S, Visser L, van der Putten A, Vlaskamp C. The interrelationships between motor, cognitive, and language development in children with and without intellectual and developmental disabilities. Res Dev Disabil. 2016;53-54:19–31. https://doi.org/10.1016/j.ridd.2016.01.012.
Kolevzon A, Gross R, Reichenberg A. Prenatal and perinatal risk factors for autism: a review and integration of findings. Arch Pediatr Adolesc Med. 2007;161:326–33. https://doi.org/10.1001/archpedi.161.4.326. AprPMID: 17404128
Sciberras E, Mulraney M, Silva D, Coghill D. Prenatal risk factors and the etiology of ADHD-review of existing evidence. Curr Psychiatry Rep. 2017;19:1. https://doi.org/10.1007/s11920-017-0753-2.
Pascal A, Govaert P, Oostra A, Naulaers G, Ortibus E, Van den Broeck C. Neurodevelopmental outcome in very preterm and very-low-birthweight infants born over the past decade: a meta-analytic review. Dev Med Child Neurol. 2018;60:342–55. https://doi.org/10.1111/dmcn.13675.
Kim SW, Youk T, Kim J. Maternal and neonatal risk factors affecting the occurrence of neurodevelopmental disorders: a population-based nationwide study. Asia Pac J Public Health. 2022;34:199–205. https://doi.org/10.1177/10105395211066383.
Ballabh P, de Vries LS. White matter injury in infants with intraventricular haemorrhage: mechanisms and therapies. Nat Rev Neurol. 2021;17:199–214. https://doi.org/10.1038/s41582-020-00447-8.
Pike K, Brocklehurst P, Jones D, Kenyon S, Salt A, Taylor D, et al. Outcomes at 7 years for babies who developed neonatal necrotising enterocolitis: the ORACLE Children Study. Arch Dis Child Fetal Neonatal Ed. 2012;97:F318–22. https://doi.org/10.1136/fetalneonatal-2011-300244.
Stoll BJ, Hansen NI, Bell EF, Shankaran S, Laptook AR, Walsh MC, et al. Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics. 2010;126:443–56. https://doi.org/10.1542/peds.2009-2959.
Obst S, Herz J, Alejandre Alcazar MA, Endesfelder S, Möbius MA, Rüdiger M, et al. Perinatal hyperoxia and developmental consequences on the lung-brain axis. Oxid Med Cell Longev. 2022;2022:5784146. https://doi.org/10.1155/2022/5784146.
Brumbaugh JE, Bell EF, Grey SF, DeMauro SB, Vohr BR, Harmon HM, et al. Eunice Kennedy Shriver National Institute of Child Health and human development neonatal research network. behavior profiles at 2 years for children born extremely preterm with bronchopulmonary dysplasia. J Pediatr. 2020;219:152–.e5. https://doi.org/10.1016/j.jpeds.2019.12.028.
Tsai WH, Hwang YS, Hung TY, Weng SF, Lin SJ, Chang WT. Association between mechanical ventilation and neurodevelopmental disorders in a nationwide cohort of extremely low birth weight infants. Res Dev Disabil. 2014;35:1544–50. https://doi.org/10.1016/j.ridd.2014.03.048.
Jensen EA, Dysart K, Gantz MG, McDonald S, Bamat NA, Keszler M, et al. The diagnosis of bronchopulmonary dysplasia in very preterm infants. an evidence-based approach. Am J Respir Crit Care Med. 2019;200:751–9. https://doi.org/10.1164/rccm.201812-2348OC.
O’Connor Leppert ML, Shank TP, Shapiro BK, Capute AJ. The Capute Scales: CAT/CLAMS—A pediatric assessment tool for the early detection of mental retardation and communicative disorders. Ment Retard Dev Disabil Res Rev 1998;4:14–19. 10.1002/(SICI)1098-2779(1998)4:1<14::AID-MRDD4>3.0.CO;2-X
J Squires, D Bricker, E Twombly, The Ages and Stages Questionnaires: SocialEmotional (ASQ: SE) User’s Guide, Paul H, Baltimore, 2002.
Robins DL, Casagrande K, Barton M, Chen CM, Dumont-Mathieu T, Fein D. Validation of the modified checklist for Autism in toddlers, revised with follow-up (M-CHAT-R/F). Pediatrics. 2014;133:37–45. https://doi.org/10.1542/peds.2013-1813.
Chlebowski C, Robins DL, Barton ML, Fein D. Large-scale use of the modified checklist for autism in low-risk toddlers. Pediatrics. 2013;131:e1121–7. https://doi.org/10.1542/peds.2012-1525.
Bose CL, Dammann CE, Laughon MM. Bronchopulmonary dysplasia and inflammatory biomarkers in the premature neonate. Arch Dis Child Fetal Neonatal Ed. 2008;93:F455–61. https://doi.org/10.1136/adc.2007.121327.
Pham K, Parikh K, Heinrich EC. Hypoxia and inflammation: insights from high-altitude physiology. Front Physiol. 2021;26:12–676782. https://doi.org/10.3389/fphys.2021.676782.
Jonsson B, Tullus K, Brauner A, Lu Y, Noack G. Early increase of TNF alpha and IL-6 in tracheobronchial aspirate fluid indicator of subsequent chronic lung disease in preterm infants. Arch Dis Child Fetal Neonatal Ed. 1997;77:F198–201.
Cayabyab RG, Jones CA, Kwong KY, Hendershott C, Lecart C, Minoo P, et al. Interleukin-1beta in the bronchoalveolar lavage fluid of premature neonates: a marker for maternal chorioamnionitis and predictor of adverse neonatal outcome. J Matern Fetal Neonatal Med. 2003;14:205–11.
Oldenburg KS, O’Shea TM, Fry RC. Genetic and epigenetic factors and early life inflammation as predictors of neurodevelopmental outcomes. Semin Fetal Neonatal Med. 2020;25:101115. https://doi.org/10.1016/j.siny.2020.101115.
Nishimura Y, Kanda Y, Sone H, Aoyama H. Oxidative stress as a common key event in developmental neurotoxicity. Oxid Med Cell Longev. 2021;2021:6685204. https://doi.org/10.1155/2021/6685204.
Wang J, Dong W. Oxidative stress and bronchopulmonary dysplasia. Gene. 2018;15:177–83. https://doi.org/10.1016/j.gene.2018.08.031.
Santos J, Pearce SE. Stroustrup A Impact of hospital-based environmental exposures on neurodevelopmental outcomes of preterm infants. Curr Opin Pediatr. 2015;27:254–60. https://doi.org/10.1097/MOP.0000000000000190.
Calafat AM, Needham LL, Silva MJ, Lambert G. Exposure to di-(2-ethylhexyl) phthalate among premature neonates in a neonatal intensive care unit. Pediatrics. 2004;113:e429–34. https://doi.org/10.1542/peds.113.5.e429.
Calafat AM, Weuve J, Ye X, Jia LT, Hu H, Ringer S, et al. Exposure to bisphenol A and other phenols in neonatal intensive care unit premature infants. Environ Health Perspect. 2009;117:639–44. https://doi.org/10.1289/ehp.0800265.
Whyatt RM, Liu X, Rauh VA, Calafat AM, Just AC, Hoepner L, et al. Maternal prenatal urinary phthalate metabolite concentrations and child mental, psychomotor, and behavioral development at 3 years of age. Environ Health Perspect. 2012;120:290–5. https://doi.org/10.1289/ehp.1103705.
Caskey M, Stephens B, Tucker R, Vohr B. Adult talk in the NICU with preterm infants and developmental outcomes. Pediatrics. 2014;133:e578–84. https://doi.org/10.1542/peds.2013-0104.
Caskey M, Stephens B, Tucker R, Vohr B. Importance of parent talk on the development of preterm infant vocalizations. Pediatrics. 2011;128:910–6. https://doi.org/10.1542/peds.2011-0609.
Reynolds LC, Duncan MM, Smith GC, Mathur A, Neil J, Inder T, et al. Parental presence and holding in the neonatal intensive care unit and associations with early neurobehavior. J Perinatol. 2013;33:636–41. https://doi.org/10.1038/jp.2013.4.
Schmidt B, Asztalos EV, Roberts RS, Robertson CM, Sauve RS, Whitfield MF. Impact of bronchopulmonary dysplasia, brain injury, and severe retinopathy on the outcome of extremely low-birth-weight infants at 18 months: results from the trial of indomethacin prophylaxis in preterms. JAMA. 2003;289:1124–9. https://doi.org/10.1001/jama.289.9.1124
Moosan H, Hoare DJ, Jayasinghe D, Willis KR, Martin K, Thornton SK. Neonatal markers of prematurity as predictors of permanent childhood hearing loss and neurodevelopmental impairment in children admitted to the neonatal intensive care unit. Brain Sci. 2024;14:926. https://doi.org/10.3390/brainsci14090926.
Author information
Authors and Affiliations
Contributions
DAR: Conceptualization, Data curation, Data collection, Formal analysis, Methodology, Writing. JG: Data curation, Data collection, Validation. AR-H: Data curation, Data collection, Validation. MW, NNP: Investigation, Data collection, Validation. ER: Data collection, Validation. KC: Formal analysis, Supervision. LK: Conceptualization, Methodology, Resources, Supervision.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This study (Pro00126633) was approved by the Medical University of South Carolina Institutional Review Board for Human Research, and informed consent was waived. All methods in this study were performed in accordance with the Declaration of Helsinki.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rueff, D.A., Gee, J., Ruddy-Humphries, A. et al. The association between bronchopulmonary dysplasia severity and neurodevelopmental disorders. J Perinatol (2026). https://doi.org/10.1038/s41372-025-02372-4
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41372-025-02372-4