Abstract
Background
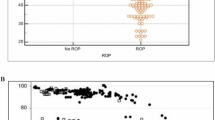
Previous studies described an association between the NRBC count at birth and risk of developing retinopathy of prematurity (ROP). Other studies correlated red blood cell (RBC) transfusions with ROP. We are aware of no studies that examined both NRBC count and RBC transfusions, in the same cohort, on ROP risk.
Study design
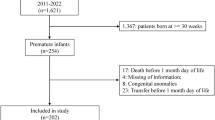
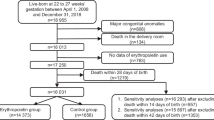
We retrospectively analyzed all infants in the Intermountain Health NICUs during the past four years who were born <32 weeks had a NRBC count at birth and had ROP examinations.
Results
Records of 386 infants demonstrated that both factors are associated with ROP. For every 1000/µL increase in NRBC, severe (grade ≥3) ROP increased by 6.8% (95% CI, 3.0–10.0%). RBC transfusions were associated with ROP incidence and severity (p = 0.001). However, neither factor alone was either necessary or sufficient for ROP.
Conclusion
The NRBC count at birth and the volume of RBC transfusions both influence ROP severity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
Data availability
Inquiries regarding data access can be addressed to the corresponding author.
Code availability
Computer code used for statistical analysis is available upon written request to the corresponding author.
References
Lubetzky R, Stolovitch C, Dollberg S, Mimouni FB, Salomon M, Mandel D. Nucleated red blood cells in preterm infants with retinopathy of prematurity. Pediatrics. 2005;116:e619–22.
Christensen RD, Henry E, Andres RL, Bennett ST. Reference ranges for blood concentrations of nucleated red blood cells in neonates. Neonatology. 2011;99:289–94.
Niranjan HS, Bharath Kumar Reddy KR, Benakappa N, Murthy K, Shivananda S, Veeranna V. Role of hematological parameters in predicting retinopathy of prematurity (ROP) in preterm neonates. Indian J Pediatr. 2013;80:726–30.
Fevereiro-Martins M, Santos AC, Marques-Neves C, Guimarães H, Bicho M. GenE-ROP Study Group. Complete blood count parameters as biomarkers of retinopathy of prematurity: a Portuguese multicenter study. Graefes Arch Clin Exp Ophthalmol. 2023;261:2997–3006.
Kaufman DA, Lucke AM, Cummings J. American Academy of Pediatrics, Committee on Fetus and Newborn. Postnatal cord blood sampling: Clinical Report. Pediatrics. 2025;155:e2025071811.
Clark C, Gibbs JAH, Maniello R, Peterbridge EW, Aranda JV. Blood transfusion: a possible risk factor in retrolental fibroplasia. Acta Pediatr Scand. 1981;70:535.
Shohat M, Reisner SH, Krikler R, Nissenkorn I, Yassur Y, Ben-Sira I. Retinopathy of prematurity: incidence and risk factors. Pediatrics. 1983;72:159–60.
Hesse L, Eberl M, Schlaud C, Poets F. Blood transfusion. Iron load and retinopathy of prematurity. Eur J Pediatr. 1997;156:465–370.
Cooke RW, Clark D, Nickey-Dwyer M, Weindling AM. The apparent role of blood transfusion in the development of retinopathy of prematurity. Eur J Pediatr. 1993;152:833–6.
Inder TE, Clemett RS, Austin NC, Graham P, Darlow BA. High iron status in very low birth weight infants is associated with an increased risk of retinopathy of prematurity. J Pediatr. 1997;131:541–4.
Collard KJ. Transfusion related morbidity in premature babies: Possible mechanisms and implications for practice. World J Clin Pediatr. 2014;3:19–29.
Lust C, Vesoulis A, Jackups R Jr, Liao S, Rao R, et al. Early red cell transfusion is associated with the development of severe retinopathy of prematurity. J Perinatol. 2019;39:393–400.
Dani C, Reali MF, Bertini G, Martelli E, Pezzati M, Rubaltelli FF. The role of blood transfusions and iron intake on retinopathy of prematurity. Early Hum Dev. 2001;62:57–63.
Del Vecchio A, Henry E, D’Amato G, Cannuscio A, Corriero L, Motta M, et al. Instituting a program to reduce the erythrocyte transfusion rate was accompanied by reductions in the incidence of bronchopulmonary dysplasia, retinopathy of prematurity and necrotizing enterocolitis. J Matern Fetal Neonatal Med. 2013;26:77–9.
Stutchfield CJ, Jain A, Odd D, Williams C, Markham R. Foetal haemoglobin, blood transfusion, and retinopathy of prematurity in very preterm infants: a pilot prospective cohort study. Eye. 2017;31:1451–5.
Zhu Z, Hua X, Yu Y, Zhu P, Hong K, Ke Y. Effect of red blood cell transfusion on the development of retinopathy of prematurity: A systematic review and meta-analysis. PLoS ONE. 2020;15:e0234266.
Hengartner T, Adams M, Pfister RE, Snyers D, McDougall J, Waldvogel S, et al. Swiss Neonatal Network. Associations between red blood cell and platelet transfusions and retinopathy of prematurity. Neonatology. 2020;117:1–7.
Dani C, Coviello C, Panin F, Frosini S, Costa S, Purcaro V, et al. Incidence and risk factors of retinopathy of prematurity in an Italian cohort of preterm infants. Ital J Pediatr. 2021;47:64.
Teofili L, Papacci P, Bartolo M, Molisso A, Orlando N, Pane L, et al. Transfusion-free survival predicts severe retinopathy in preterm neonates. Front Pediatr. 2022;10:814194.
Raffa LH, Aljohani W. Evaluation of the effect of blood transfusion on retinopathy of prematurity at a tertiary care center in western Saudi Arabia. Cureus. 2022;14:e24495.
Teofili L, Papacci P, Pellegrino C, Dani C, Cresi F, Remachi G, et al. Cord red blood cell transfusions for severe retinopathy in preterm neonates in Italy: a multicenter randomized controlled trial. eClinicalMedicine. 2025;87:103426.
Prasad N, Dubey A, Kumar K, Shrivastava J. Role of fetal hemoglobin in the development and progression of retinopathy of prematurity in preterm infants. Indian J Ophthalmol. 2023;71:3478–83.
Glaser K, Härtel C, Dammann O, Herting E, Andres O, Speer CP, et al. German Neonatal Network. Erythrocyte transfusions are associated with retinopathy of prematurity in extremely low gestational age newborns. Acta Paediatr. 2023;112:2507–15.
Torrejon-Rodriguez L, Pinilla-Gonzalez A, Lara Cantón I, Albiach-Delgado A, Cascant-Vilaplana MM, Cernada M, et al. Effect of autologous umbilical cord blood transfusion in the development of retinopathy of prematurity: randomized clinical trial - study protocol. Front Pediatr. 2023;11:1269797.
Pellegrino C, Papacci P, Beccia F, Serrao F, Cantone GV, Cannetti G, et al. Differences in cerebral tissue oxygenation in preterm neonates receiving adult or cord blood red cell transfusions. JAMA Netw Open. 2023;6:e2341643.
Teofili L, Papacci P, Dani C, Cresi F, Remaschi G, Pellegrino C, et al. Cord blood transfusions in extremely low gestational age neonates to reduce severe retinopathy of prematurity: results of a prespecified interim analysis of the randomized BORN trial. Ital J Pediatr. 2024;50:142.
El Emrani S, van der Meeren LE, Lopriore E, Schalij-Delfos NE. Erythrocyte transfusions and retinopathy of prematurity: plea for application of the two-phase theory. Acta Paediatrica. 2024;113:615–6.
Wang X, Rao R, Li H, Lei X, Dong W. Red blood cell transfusion for incidence of retinopathy of prematurity: prospective multicenter cohort study. JMIR Pediatr Parent. 2024;7:e60330.
Fevereiro-Martins M, Santos AC, Marques-Neves C, Bicho M, Guimarães H, On Behalf Of The GenE-Rop Study Group. Retinopathy of prematurity in eight Portuguese neonatal intensive care units: incidence, risk factors, and progression-a prospective multicenter study. Children. 2024;11:1154.
Fevereiro-Martins M, Aguiar L, Inácio Â, Cardoso C, Santos AC, Marques-Neves C, et al. GenE-ROP Study Group. Fetal hemoglobin as a predictive biomarker for retinopathy of prematurity: a prospective multicenter cohort study in portugal. Biomedicines. 2025;13:110.
El Emrani S, Derks LA, Tjiam AM, van Bohemen M, Termote JUM, van der Meeren LE, et al. The association between red blood cell transfusion timing and the development of retinopathy of prematurity: Application of the two-phase theory. Acta Ophthalmol. 2025. https://doi.org/10.1111/aos.17471.
Chiang MF, Quinn GE, Fielder AR, Ostmo SR, Paul Chan RV, Berrocal A, et al. International Classification of Retinopathy of Prematurity, Third Edition. Ophthalmology. 2021;128:e51–e68.
Fierson WM, AAP, AAO, AAPOS, AACO, Chiang MF, et al. Screening examination of premature infants for retinopathy of prematurity. Pediatrics 2018:142: e20183061.
Tweddell SM, Morelli TM, Bahr TM, Krueger A, Christensen RD, Ohls RK Identifying barriers to complying with new restrictive NICU transfusion guidelines. J Perinatol. 2025 Feb 16. https://doi.org/10.1038/s41372-025-02225-0. Epub ahead of print.
Christensen RD, Bahr TM, Davenport P, Sola-Visner MC, Kelley WE, Ilstrup SJ, et al. Neonatal thrombocytopenia: factors associated with the platelet count increment following platelet transfusion. J Pediatr. 2023;263:113666.
Altman N, Krzywinski M. Association, correlation and causation. Nature Methods. 2015;12:899–900.
Buonocore G, Perrone S, Gioia D, Gatti MG, Massafra C, Agosta R, et al. Nucleated red blood cell count at birth as an index of perinatal brain damage. Am J Obstet Gynecol. 1999;181:1500–5.
Perrone S, Bracci R, Buonocore G. New biomarkers of fetal-neonatal hypoxic stress. Acta Paediatr Suppl. 2002;91:135–8.
Bahr TM, Henry E, O’Brien EA, Christensen RD. Nucleated red blood cell counts of neonates born emergently 1-4 h after a maternal cardiac arrest. Neonatology. 2022;119:255–9.
Dammann O, Hartnett ME, Stahl A. Retinopathy of prematurity. Dev Med Child Neurol. 2023;65:625–31.
Bishnoi K, Prasad R, Upadhyay T, Mathurkar S. A narrative review on managing retinopathy of prematurity: insights into pathogenesis, screening, and treatment strategies. Cureus. 2024;16:e56168.
Owen LA, Hartnett ME. Current concepts of oxygen management in retinopathy of prematurity. J Ophthalmic Vis Res. 2014;9:94–100. Jan.
Dammann O, Stansfield BK. Neonatal sepsis as a cause of retinopathy of prematurity: An etiological explanation. Prog Retin Eye Res. 2024;98:101230.
Katheria AC, Lee HC. EBNEO Commentary on ‘physiological versus time based cord clamping in very preterm infants (ABC3): A parallel-group, multicentre, randomized, controlled superiority trial’. Acta Paediatr. 2025;114:1744–5.
Deschmann E, Dame C, Sola-Visner MC, Fustolo-Gunnink SF, Guyatt GH, Patel RM, et al. Neonatal Transfusion Network. Clinical practice guideline for red blood cell transfusion thresholds in very preterm neonates. JAMA Netw Open. 2024;7:e2417431.
Christensen RD, Bahr TM, Ohls RK. Administering supplemental iron and erythropoiesis-stimulating agents to infants born preterm: what do we need to build consensus?. J Pediatr. 2025;279:114460.
Ohls RK, Bahr TM, Peterson TG, Christensen RD. A practical guide to reducing/eliminating red blood cell transfusions in the neonatal intensive care unit. Semin Fetal Neonatal Med. 2025;30:101545.
Christensen RD, Bahr TM, Christensen TR, Ohls RK, Krong J, Carlton LC, et al. Banked term umbilical cord blood to meet the packed red blood cell transfusion needs of extremely-low-gestational-age neonates: a feasibility analysis. J Perinatol. 2024;44:873–9.
Bahr TM, Ohls TM, Christensen TR, Monson MC, Page JM, Dabling HA, et al. Three studies needed to inform the design of the U-BET (umbilical cord blood for extremely low-gestational-age transfusions) clinical trial. J Perinatol. 2025 Jul 28. https://doi.org/10.1038/s41372-025-02345-7. Online ahead of print.
Bahr TM, Christensen TR, Ilstrup SJ, Ohls RK, Christensen RD. Term umbilical cord blood, fully tested and processed, as the source of red blood cell transfusions for extremely-low-gestational age neonates. Semin Fetal Neonatal Med. 2025;30:101546.
Ohls RK, Bahr TM, Kaufman DA, Christensen RD. A survey of level 3 NICUs in Utah to the June 2025 American Academy of Pediatrics Committee on Fetus and Newborn clinical report on postnatal cord blood sampling. J Perinatol. 2025. https://doi.org/10.1038/s41372-025-02418-7 Epub ahead of print.
Author information
Authors and Affiliations
Contributions
Conceptualization, TMB, KD, RKO, RDC, Methodology, TMB, KD, RDC, Original Draft Preparation, RDC, Review and Editing, BZ, TMB, KD, MEH, SJI, RKO, RDC.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
The protocol for conducting this retrospective, deidentified, records review was approved by the Intermountain Health Institutional Review Board with a waiver of informed consent (IRB # 1051715). All methods were performed in accordance with United States Department of Health and Human Services regulations, including the Health Insurance Portability and Accountability Act (HIPAA) and the Federal Policy for the Protection of Human Subjects (Common Rule, 45 CFR 46).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zeiler, B., Bahr, T.M., Esplin, S.D. et al. The nucleated red blood cell count at birth, the volume of red cell transfusions received, and the risk of developing retinopathy of prematurity. J Perinatol 45, 1809–1815 (2025). https://doi.org/10.1038/s41372-025-02519-3
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41372-025-02519-3