Abstract
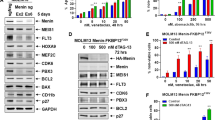
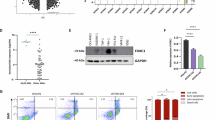
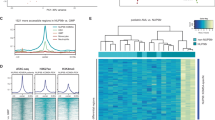
Chromosomal translocations of the nucleoporin 98 (NUP98) gene are found in acute myeloid leukemia (AML) patients leading to very poor outcomes. The oncogenic activity of NUP98 fusion proteins is dependent on the interaction between Mixed Lineage Leukemia 1 and menin. NUP98-rearranged (NUP98-r) leukemia cells also rely on specific kinases, including CDK6 and/or FLT3, suggesting that simultaneous targeting of these kinases and menin could overcome limited sensitivity to single agents. Here, we found that combinations of menin inhibitor, MI-3454, with kinase inhibitors targeting either CDK6 (Palbociclib) or FLT3 (Gilteritinib) strongly enhance the anti-leukemic effect of menin inhibition in NUP98-r leukemia models. We found strong synergistic effects of both combinations on cell growth, colony formation and differentiation in patient samples with NUP98 translocations. These combinations also markedly augmented anti-leukemic efficacy of menin inhibitor in Patient Derived Xenograft models of NUP98-r leukemia. Despite inhibiting two unrelated kinases, when Palbociclib or Gilteritinib were combined with the menin inhibitor, they affected similar pathways relevant to leukemogenesis, including cell cycle regulation, cell proliferation and differentiation. This study provides strong rationale for clinical translation of the combination of menin and kinase inhibitors as novel treatments for NUP98-r leukemia, supporting the unexplored combinations of epigenetic drugs with kinase inhibitors.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The datasets generated and analyzed during the current study are available from the corresponding authors on reasonable request.
References
Michmerhuizen NL, Klco JM, Mullighan CG. Mechanistic insights and potential therapeutic approaches for NUP98-rearranged hematologic malignancies. Blood. 2020;136:2275–2289.
Gough SM, Slape CI, Aplan PD. NUP98 gene fusions and hematopoietic malignancies: common themes and new biologic insights. Blood. 2011;118:6247–6257.
Bisio V, Zampini M, Tregnago C, Manara E, Salsi V, Di Meglio A, et al. NUP98-fusion transcripts characterize different biological entities within acute myeloid leukemia: a report from the AIEOP-AML group. Leukemia. 2017;31:974–977.
Hollink IH, van den Heuvel-Eibrink MM, Arentsen-Peters ST, Pratcorona M, Abbas S, Kuipers JE, et al. NUP98/NSD1 characterizes a novel poor prognostic group in acute myeloid leukemia with a distinct HOX gene expression pattern. Blood. 2011;118:3645–3656.
Marceau-Renaut A, Duployez N, Ducourneau B, Labopin M, Petit A, Rousseau A, et al. Molecular Profiling Defines Distinct Prognostic Subgroups in Childhood AML: A Report From the French ELAM02 Study Group. Hemasphere. 2018;2:e31.
Thol F, Kolking B, Hollink IH, Damm F, van den Heuvel-Eibrink MM, Michel Zwaan C, et al. Analysis of NUP98/NSD1 translocations in adult AML and MDS patients. Leukemia. 2013;27:750–754.
Wang GG, Cai L, Pasillas MP, Kamps MP. NUP98-NSD1 links H3K36 methylation to Hox-A gene activation and leukaemogenesis. Nat Cell Biol. 2007;9:804–812.
Ostronoff F, Othus M, Gerbing RB, Loken MR, Raimondi SC, Hirsch BA, et al. NUP98/NSD1 and FLT3/ITD coexpression is more prevalent in younger AML patients and leads to induction failure: a COG and SWOG report. Blood. 2014;124:2400–2407.
Niktoreh N, Walter C, Zimmermann M, von Neuhoff C, von Neuhoff N, Rasche M, et al. Mutated WT1, FLT3-ITD, and NUP98-NSD1 Fusion in Various Combinations Define a Poor Prognostic Group in Pediatric Acute Myeloid Leukemia. J Oncol. 2019;2019:1609128.
Xu H, Valerio DG, Eisold ME, Sinha A, Koche RP, Hu W, et al. NUP98 Fusion Proteins Interact with the NSL and MLL1 Complexes to Drive Leukemogenesis. Cancer Cell. 2016;30:863–878.
de Rooij JD, Hollink IH, Arentsen-Peters ST, van Galen JF, Berna Beverloo H, Baruchel A, et al. NUP98/JARID1A is a novel recurrent abnormality in pediatric acute megakaryoblastic leukemia with a distinct HOX gene expression pattern. Leukemia. 2013;27:2280–2288.
Heikamp EB, Henrich JA, Perner F, Wong EM, Hatton C, Wen Y, et al. The menin-MLL1 interaction is a molecular dependency in NUP98-rearranged AML. Blood. 2022;139:894–906.
Rasouli M, Blair H, Troester S, Szoltysek K, Cameron R, Ashtiani M, et al. The MLL-Menin Interaction is a Therapeutic Vulnerability in NUP98-rearranged AML. Hemasphere. 2023;7:e935.
Yokoyama A, Somervaille TC, Smith KS, Rozenblatt-Rosen O, Meyerson M, Cleary ML. The menin tumor suppressor protein is an essential oncogenic cofactor for MLL-associated leukemogenesis. Cell. 2005;123:207–218.
Yokoyama A, Cleary ML. Menin critically links MLL proteins with LEDGF on cancer-associated target genes. Cancer Cell. 2008;14:36–46.
Caslini C, Yang Z, El-Osta M, Milne TA, Slany RK, Hess JL. Interaction of MLL amino terminal sequences with menin is required for transformation. Cancer Res. 2007;67:7275–7283.
Kuhn MW, Song E, Feng Z, Sinha A, Chen CW, Deshpande AJ, et al. Targeting Chromatin Regulators Inhibits Leukemogenic Gene Expression in NPM1 Mutant Leukemia. Cancer Discov. 2016;6:1166–1181.
Grembecka J, He S, Shi A, Purohit T, Muntean AG, Sorenson RJ, et al. Menin-MLL inhibitors reverse oncogenic activity of MLL fusion proteins in leukemia. Nat Chem Biol. 2012;8:277–284.
Borkin D, He S, Miao H, Kempinska K, Pollock J, Chase J, et al. Pharmacologic inhibition of the Menin-MLL interaction blocks progression of MLL leukemia in vivo. Cancer Cell. 2015;27:589–602.
Borkin D, Pollock J, Kempinska K, Purohit T, Li X, Wen B, et al. Property Focused Structure-Based Optimization of Small Molecule Inhibitors of the Protein-Protein Interaction between Menin and Mixed Lineage Leukemia (MLL). J Med Chem. 2016;59:892–913.
Borkin D, Klossowski S, Pollock J, Miao H, Linhares BM, Kempinska K, et al. Complexity of Blocking Bivalent Protein-Protein Interactions: Development of a Highly Potent Inhibitor of the Menin-Mixed-Lineage Leukemia Interaction. J Med Chem. 2018;61:4832–4850.
Shi A, Murai MJ, He S, Lund G, Hartley T, Purohit T, et al. Structural insights into inhibition of the bivalent menin-MLL interaction by small molecules in leukemia. Blood. 2012;120:4461–4469.
Klossowski S, Miao H, Kempinska K, Wu T, Purohit T, Kim E, et al. Menin inhibitor MI-3454 induces remission in MLL1-rearranged and NPM1-mutated models of leukemia. J Clin Invest. 2020;130:981–997.
Miao H, Kim E, Chen D, Purohit T, Kempinska K, Ropa J, et al. Combinatorial treatment with menin and FLT3 inhibitors induces complete remission in AML models with activating FLT3 mutations. Blood. 2020;136:2958–2963.
Erba HP, Fathi AT, Issa GC, Altman JK, Montesinos P, Patnaik MM, et al. Update on a Phase 1/2 First-in-Human Study of the Menin-KMT2A (MLL) Inhibitor Ziftomenib (KO-539) in Patients with Relapsed or Refractory Acute Myeloid Leukemia. Blood. 2022;140:153–156.
Dempke WCM, Desole M, Chiusolo P, Sica S, Schmidt-Hieber M. Targeting the undruggable: menin inhibitors ante portas. J Cancer Res Clin Oncol. 2023;149:9451–9459.
Schmoellerl J, Barbosa IAM, Eder T, Brandstoetter T, Schmidt L, Maurer B, et al. CDK6 is an essential direct target of NUP98 fusion proteins in acute myeloid leukemia. Blood. 2020;136:387–400.
Chou TC. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010;70:440–446.
Fiskus W, Boettcher S, Daver N, Mill CP, Sasaki K, Birdwell CE, et al. Effective Menin inhibitor-based combinations against AML with MLL rearrangement or NPM1 mutation (NPM1c). Blood Cancer J. 2022;12:5.
Oyelade J, Isewon I, Oladipupo F, Aromolaran O, Uwoghiren E, Ameh F, et al. Clustering Algorithms: Their Application to Gene Expression Data. Bioinform Biol Insights. 2016;10:237–253.
Whiteford CC, Bilke S, Greer BT, Chen Q, Braunschweig TA, Cenacchi N, et al. Credentialing preclinical pediatric xenograft models using gene expression and tissue microarray analysis. Cancer Res. 2007;67:32–40.
Prescott JE, Osthus RC, Lee LA, Lewis BC, Shim H, Barrett JF, et al. A novel c-Myc-responsive gene, JPO1, participates in neoplastic transformation. J Biol Chem. 2001;276:48276–48284.
Gillespie M, Jassal B, Stephan R, Milacic M, Rothfels K, Senff-Ribeiro A, et al. The reactome pathway knowledgebase 2022. Nucleic Acids Res. 2022;50:D687–D692.
Hess JL, Bittner CB, Zeisig DT, Bach C, Fuchs U, Borkhardt A, et al. c-Myb is an essential downstream target for homeobox-mediated transformation of hematopoietic cells. Blood. 2006;108:297–304.
Issa GC, Aldoss I, DiPersio J, Cuglievan B, Stone R, Arellano M, et al. The menin inhibitor revumenib in KMT2A-rearranged or NPM1-mutant leukaemia. Nature. 2023;615:920–924.
Perner F, Stein EM, Wenge DV, Singh S, Kim J, Apazidis A, et al. MEN1 mutations mediate clinical resistance to menin inhibition. Nature. 2023;615:913–919.
Dzama MM, Steiner M, Rausch J, Sasca D, Schonfeld J, Kunz K, et al. Synergistic targeting of FLT3 mutations in AML via combined menin-MLL and FLT3 inhibition. Blood. 2020;136:2442–2456.
Negotei C, Colita A, Mitu I, Lupu AR, Lapadat ME, Popovici CE, et al. A Review of FLT3 Kinase Inhibitors in AML. J Clin Med. 2023;12:6428.
Takahashi S. Combination Therapies with Kinase Inhibitors for Acute Myeloid Leukemia Treatment. Hematol Rep. 2023;15:331–346.
Megias-Vericat JE, Ballesta-Lopez O, Barragan E, Martinez-Cuadron D, Montesinos P. Tyrosine kinase inhibitors for acute myeloid leukemia: A step toward disease control? Blood Rev. 2020;44:100675.
Alharbi RA, Pettengell R, Pandha HS, Morgan R. The role of HOX genes in normal hematopoiesis and acute leukemia. Leukemia. 2013;27:1000–1008.
Ben-Porath I, Thomson MW, Carey VJ, Ge R, Bell GW, Regev A, et al. An embryonic stem cell-like gene expression signature in poorly differentiated aggressive human tumors. Nat Genet. 2008;40:499–507.
Acknowledgements
We thank Drs. Gwenn Danet-Desnoyers and Martin Carroll from the Stem Cell and Xenograft Core at the University of Pennsylvania for providing patient samples for these studies. We thank Joshua Ray and Sydney Musser for critical reading of this manuscript. The mouse work was performed under oversight of UCUCA at the University of Michigan. This work was funded by the National Institute of Health (NIH) R01 grants (1R01CA160467, 1R01CA272561 and R01CA244254) to JG, NIH R01 grants (1R01 CA226759 and 1R01 CA282082) to TC, NIH F32 (F31HL160072) and NIH K99 (K99HL166790) to JR, LLS TRP (6649-23) to JG, ALSF Reach grant (22-25561) to J.G., and Rogel Scholar grants to JG and TC.
Author information
Authors and Affiliations
Contributions
HM, DC and JR contributed equally to this work. DC designed and synthesized compounds. HM designed and performed in vivo efficacy studies and biological studies in leukemia cell lines and primary patient samples. JR analyzed RNA-seq data. TP performed cell biology experiments. EK performed in vivo efficacy studies. AF and M-LS supported the project with primary patient samples and interpreted data. JG and TC directed the entire project, designed the experiments, analyzed the results and wrote the manuscript with the input from all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare the following competing financial interest(s): JG and TC received research support from Kura Oncology, Inc. They have also served as consultants for Kura Oncology, have equity ownership in the company and are co-inventors on patent applications covering MI-3454. Kura Oncology, Inc. and University of Michigan have filed patent applications covering MI-3454 and they hold intellectual property rights on this compound. HM is co-inventor on patent applications covering MI-3454 or related compounds, which were licensed by Kura Oncology. JG, TC and HM receive royalties from the University of Michigan on the patents covering menin inhibitors that were licensed to Kura Oncology. AF is an employee of Regeneron Pharmaceuticals. Other co-authors declare no potential conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Miao, H., Chen, D., Ropa, J. et al. Combination of menin and kinase inhibitors as an effective treatment for leukemia with NUP98 translocations. Leukemia 38, 1674–1687 (2024). https://doi.org/10.1038/s41375-024-02312-9
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41375-024-02312-9
This article is cited by
-
Inhibition of EYA family tyrosine phosphatase activity reveals a therapeutic vulnerability and enhances Menin and DOT1L inhibitor efficacy in KMT2A-rearranged leukemia
Experimental Hematology & Oncology (2025)
-
One step further in targeting acute leukemia by combining antibody-based immunotherapies and small molecule inhibitors
Cancer Cell International (2025)