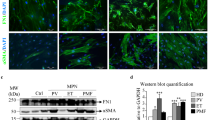
Abstract
Despite increased understanding of the genomic landscape of Myeloproliferative Neoplasms (MPNs), the pathological mechanisms underlying abnormal megakaryocyte (Mk)-stromal crosstalk and fibrotic progression in MPNs remain unclear. We conducted mass spectrometry-based proteomics on mice with Romiplostim-dependent myelofibrosis to reveal alterations in signaling pathways and protein changes in Mks, platelets, and bone marrow (BM) cells. The chemokine Platelet Factor 4 (PF4)/Cxcl4 was up-regulated in all proteomes and increased in plasma and BM fluids of fibrotic mice. High TPO concentrations sustained in vitro PF4 synthesis and secretion in cultured Mks, while Ruxolitinib restrains the abnormal PF4 expression in vivo. We discovered that PF4 is rapidly internalized by stromal cells through surface glycosaminoglycans (GAGs) to promote myofibroblast differentiation. Cxcl4 gene silencing in Mks mitigated the profibrotic phenotype of stromal cells in TPO-saturated co-culture conditions. Consistently, extensive stromal PF4 uptake and altered GAGs deposition were detected in Romiplostim-treated, JAK2V617F mice and BM biopsies of MPN patients. BM PF4 levels and Mk/platelet CXCL4 expression were elevated in patients, exclusively in overt fibrosis. Finally, pharmacological inhibition of GAGs ameliorated in vivo fibrosis in Romiplostim-treated mice. Thus, our findings highlight the critical role of PF4 in the fibrosis progression of MPNs and substantiate the potential therapeutic strategy of neutralizing PF4-GAGs interaction.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout







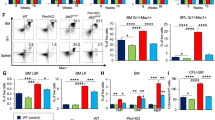
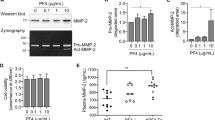
Similar content being viewed by others
Data availability
Proteomic data have been deposited to the UNIMI Dataverse repository, accessible at: https://doi.org/10.13130/RD_UNIMI/RI3IDJ.
Change history
27 March 2025
The original online version of this article was revised: In this article the author’s name Niccolò Bartalucci was incorrectly written as Bartalucci Niccolò. The original article has been corrected.
01 April 2025
A Correction to this paper has been published: https://doi.org/10.1038/s41375-025-02587-6
References
Malara A, Balduini A. Blood platelet production and morphology. Thrombosis Res. 2012;129:241–4.
Patel SR, Hartwig JH, Italiano JE. The biogenesis of platelets from megakaryocyte proplatelets. J Clin Invest. 2005;115:3348–54.
Lok S, Kaushansky K, Holly RD, Kuijper JL, Lofton-Day CE, Oort PJ, et al. Cloning and expression of murine thrombopoietin cDNA and stimulation of platelet production in vivo. Nature. 1994;369:565–8.
Bruns I, Lucas D, Pinho S, Ahmed J, Lambert MP, Kunisaki Y, et al. Megakaryocytes regulate hematopoietic stem cell quiescence through CXCL4 secretion. Nat Med. 2014;20:1315–20.
Avecilla ST, Hattori K, Heissig B, Tejada R, Liao F, Shido K, et al. Chemokine-mediated interaction of hematopoietic progenitors with the bone marrow vascular niche is required for thrombopoiesis. Nat Med. 2004;10:64–71.
Malara A, Currao M, Gruppi C, Celesti G, Viarengo G, Buracchi C, et al. Megakaryocytes contribute to the bone marrow-matrix environment by expressing fibronectin, type IV collagen, and laminin. Stem Cells. 2014;32:926–37.
Wang J, Xie J, Wang D, Han X, Chen M, Shi G, et al. CXCR4high megakaryocytes regulate host-defense immunity against bacterial pathogens. Elife. 2022;11:e78662.
Sun S, Jin C, Si J, Lei Y, Chen K, Cui Y, et al. Single-cell analysis of ploidy and the transcriptome reveals functional and spatial divergency in murine megakaryopoiesis. Blood. 2021;138:1211–24.
Woods B, Chen W, Chiu S, Marinaccio C, Fu C, Gu L, et al. Activation of JAK/STAT signaling in megakaryocytes sustains myeloproliferation. Clin Cancer Res. 2019;25:5901–12.
Vardiman JW, Thiele J, Arber DA, Brunning RD, Borowitz MJ, Porwit A, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114:937–51.
Vannucchi AM, Guglielmelli P, Tefferi A. Advances in understanding and management of myeloproliferative neoplasms. CA Cancer J Clin. 2009;59:171–91.
Waksal JA, Mascarenhas J. Novel therapies in myelofibrosis: beyond JAK inhibitors. Curr Hematol Malig Rep. 2022;17:140–54.
James C, Ugo V, Le Couédic JP, Staerk J, Delhommeau F, Lacout C, et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature. 2005;434:1144–8.
Pikman Y, Lee BH, Mercher T, McDowell E, Ebert BL, Gozo M, et al. MPLW515L is a novel somatic activating mutation in myelofibrosis with myeloid metaplasia. PLoS Med. 2006;3:e270.
Nangalia J, Massie CE, Baxter EJ, Nice FL, Gundem G, Wedge DC, et al. Somatic CALR mutations in myeloproliferative neoplasms with nonmutated JAK2. N. Engl J Med. 2013;369:2391–405.
Hasselbalch HC, Bjørn ME. MPNs as inflammatory diseases: the evidence, consequences, and perspectives. Mediat Inflamm. 2015;2015:102476.
Malara A, Gruppi C, Abbonante V, Cattaneo D, De Marco L, Massa M, et al. EDA fibronectin-TLR4 axis sustains megakaryocyte expansion and inflammation in bone marrow fibrosis. J Exp Med. 2019;216:587–604.
Domon B, Aebersold R. Options and considerations when selecting a quantitative proteomics strategy. Nat Biotechnol. 2010;28:710–21.
Hasselbalch HC. The role of cytokines in the initiation and progression of myelofibrosis. Cytokine Growth Factor Rev. 2013;24:133–45.
Malara A, Abbonante V, Zingariello M, Migliaccio A, Balduini A. Megakaryocyte contribution to bone marrow fibrosis: many arrows in the quiver. Mediterr J Hematol Infect Dis. 2018;10:e2018068.
Lambert MP, Wang Y, Bdeir KH, Nguyen Y, Kowalska MA, Poncz M. Platelet factor 4 regulates megakaryopoiesis through low-density lipoprotein receptor-related protein 1 (LRP1) on megakaryocytes. Blood. 2009;114:2290–8.
Lasagni L, Francalanci M, Annunziato F, Lazzeri E, Giannini S, Cosmi L, et al. An alternatively spliced variant of CXCR3 mediates the inhibition of endothelial cell growth induced by IP-10, Mig, and I-TAC, and acts as functional receptor for platelet factor 4. J Exp Med. 2003;197:1537–49.
Le HT, Golla K, Karimi R, Hughes MR, Lakschevitz F, Cines DB, et al. Platelet factor 4 (CXCL4/PF4) upregulates matrix metalloproteinase-2 (MMP-2) in gingival fibroblasts. Sci Rep. 2022;12:18636.
Kai Y, Yoneyama H, Koyama J, Hamada K, Kimura H, Matsushima K. Treatment with chondroitinase ABC alleviates bleomycin-induced pulmonary fibrosis. Med Mol Morphol. 2007;40:128–40.
Zou XH, Foong WC, Cao T, Bay BH, Ouyang HW, Yip GW. Chondroitin sulfate in palatal wound healing. J Dent Res. 2004;83:880–5.
Schneider RK, Mullally A, Dugourd A, Peisker F, Hoogenboezem R, Van Strien PMH, et al. Gli1+ Mesenchymal Stromal Cells Are a Key Driver of Bone Marrow Fibrosis and an Important Cellular Therapeutic Target. Cell Stem Cell. 2017;20:785–800.e8.
Ozono Y, Shide K, Kameda T, Kamiunten A, Tahira Y, Sekine M, et al. Neoplastic fibrocytes play an essential role in bone marrow fibrosis in Jak2V617F-induced primary myelofibrosis mice. Leukemia. 2021;35:454–67.
Verstovsek S, Manshouri T, Pilling D, Bueso-Ramos CE, Newberry KJ, Prijic S, et al. Role of neoplastic monocyte-derived fibrocytes in primary myelofibrosis. J Exp Med. 2016;213:1723–40.
Decker M, Martinez-Morentin L, Wang G, Lee Y, Liu Q, Leslie J, et al. Leptin-receptor-expressing bone marrow stromal cells are myofibroblasts in primary myelofibrosis. Nat Cell Biol. 2017;19:677–88.
Hasan S, Lacout C, Marty C, Cuingnet M, Solary E, Vainchenker W, et al. JAK2V617F expression in mice amplifies early hematopoietic cells and gives them a competitive advantage that is hampered by IFNα. Blood. 2013;122:1464–77.
Koschmieder S, Mughal TI, Hasselbalch HC, Barosi G, Valent P, Kiladjian JJ, et al. Myeloproliferative neoplasms and inflammation: whether to target the malignant clone or the inflammatory process or both. Leukemia. 2016;30:1018–24.
Tefferi A, Vaidya R, Caramazza D, Finke C, Lasho T, Pardanani A. Circulating interleukin (IL)-8, IL-2R, IL-12, and IL-15 levels are independently prognostic in primary myelofibrosis: a comprehensive cytokine profiling study. J Clin Oncol. 2011;29:1356–63.
Abbonante V, Di Buduo CA, Gruppi C, Malara A, Gianelli U, Celesti G, et al. Thrombopoietin/TGF-?1 loop regulates megakaryocyte extracellular matrix component synthesis. Stem Cells. 2016;34:1123–33.
Ceglia I, Dueck AC, Masiello F, Martelli F, He W, Federici G, et al. Preclinical rationale for TGF-β inhibition as a therapeutic target for the treatment of myelofibrosis. Exp Hematol. 2016;44:1138–55.e4.
Chagraoui H, Komura E, Tulliez M, Giraudier S, Vainchenker W, Wendling F. Prominent role of TGF-beta 1 in thrombopoietin-induced myelofibrosis in mice. Blood. 2002;100:3495–503.
Zingariello M, Martelli F, Ciaffoni F, Masiello F, Ghinassi B, D’Amore E, et al. Characterization of the TGF-β1 signaling abnormalities in the Gata1low mouse model of myelofibrosis. Blood. 2013;121:3345–63.
Bock O, Loch G, Büsche G, von Wasielewski R, Schlué J, Kreipe H. Aberrant expression of platelet-derived growth factor (PDGF) and PDGF receptor-alpha is associated with advanced bone marrow fibrosis in idiopathic myelofibrosis. Haematologica. 2005;90:133–4.
Ciurea SO, Merchant D, Mahmud N, Ishii T, Zhao Y, Hu W, et al. Pivotal contributions of megakaryocytes to the biology of idiopathic myelofibrosis. Blood. 2007;110:986–93.
Zhan H, Ma Y, Lin CH, Kaushansky K. JAK2V617F-mutant megakaryocytes contribute to hematopoietic stem/progenitor cell expansion in a model of murine myeloproliferation. Leukemia. 2016;30:2332–41.
Shen Z, Du W, Perkins C, Fechter L, Natu V, Maecker H, et al. Platelet transcriptome identifies progressive markers and potential therapeutic targets in chronic myeloproliferative neoplasms. Cell Rep. Med. 2021;2:100425.
Jain K, Tyagi T, Du J, Hu X, Patell K, Martin KA, et al. Unfolded protein response differentially modulates the platelet phenotype. Circ Res. 2022;131:290–307.
Mbiandjeu S, Balduini A, Malara A. Megakaryocyte cytoskeletal proteins in platelet biogenesis and diseases. Thromb Haemost. 2022;122:666–78.
Ghalloussi D, Dhenge A, Bergmeier W. New insights into cytoskeletal remodeling during platelet production. J Thromb Haemost. 2019;17:1430–9.
Poulter NS, Thomas SG. Cytoskeletal regulation of platelet formation: coordination of F-actin and microtubules. Int J Biochem Cell Biol. 2015;66:69–74.
Chang Y, Auradé F, Larbret F, Zhang Y, Le Couedic JP, Momeux L, et al. Proplatelet formation is regulated by the Rho/ROCK pathway. Blood. 2007;109:4229–36.
Murphy-Ullrich JE. Thrombospondin 1 and its diverse roles as a regulator of extracellular matrix in fibrotic disease. J Histochem Cytochem. 2019;67:683–99.
Muth M, Engelhardt BM, Kröger N, Hussein K, Schlué J, Büsche G, et al. Thrombospondin-1 (TSP-1) in primary myelofibrosis (PMF) - a megakaryocyte-derived biomarker which largely discriminates PMF from essential thrombocythemia. Ann Hematol. 2011;90:33–40.
Evrard S, Bluteau O, Tulliez M, Rameau P, Gonin P, Zetterberg E, et al. Thrombospondin-1 is not the major activator of TGF-β1 in thrombopoietin-induced myelofibrosis. Blood. 2011;117:246–9.
Leimkühler NB, Gleitz HFE, Ronghui L, Snoeren IAM, Fuchs SNR, Nagai JS, et al. Heterogeneous bone-marrow stromal progenitors drive myelofibrosis via a druggable alarmin axis. Cell Stem Cell. 2021;28:637–52.e8.
Affandi AJ, Carvalheiro T, Ottria A, de Haan JJ, Brans MAD, Brandt MM, et al. CXCL4 drives fibrosis by promoting several key cellular and molecular processes. Cell Rep. 2022;38:110189.
Buka RJ, Montague SJ, Moran LA, Martin EM, Slater A, Watson SP, et al. PF4 activates the c-Mpl-Jak2 pathway in platelets. Blood. 2024;143:64–9.
Gleitz HFE, Dugourd AJF, Leimkühler NB, Snoeren IAM, Fuchs SNR, Menzel S, et al. Increased CXCL4 expression in hematopoietic cells links inflammation and progression of bone marrow fibrosis in MPN. Blood. 2020;136:2051–64.
Martinaud C, Desterke C, Konopacki J, Pieri L, Torossian F, Golub R, et al. Osteogenic potential of mesenchymal stromal cells contributes to primary myelofibrosis. Cancer Res. 2015;75:4753–65.
Korf-Klingebiel M, Reboll MR, Grote K, Schleiner H, Wang Y, Wu X, et al. Heparan sulfate-editing extracellular sulfatases enhance VEGF bioavailability for ischemic heart repair. Circ Res. 2019;125:787–801.
Warford JR, Lamport AC, Clements DR, Malone A, Kennedy BE, Kim Y, et al. Surfen, a proteoglycan binding agent, reduces inflammation but inhibits remyelination in murine models of Multiple Sclerosis. Acta Neuropathol Commun. 2018;6:4.
Thompson S, Martínez-Burgo B, Sepuru KM, Rajarathnam K, Kirby JA, Sheerin NS, et al. Regulation of chemokine function: the roles of GAG-binding and post-translational nitration. Int J Mol Sci. 2017;18:1692.
Leoni P, Rupoli S, Lai G, Brunelli MA, Belmonte MM, Pugnaloni A, et al. Platelet abnormalities in idiopathic myelofibrosis: functional, biochemical and immunomorphological correlations. Haematologica. 1994;79:29–39.
Małecki R, Gacka M, Kuliszkiewicz-Janus M, Jakobsche-Policht U, Kwiatkowski J, Adamiec R, et al. Altered plasma fibrin clot properties in essential thrombocythemia. Platelets. 2016;27:110–6.
Meier-Abt F, Wolski WE, Tan G, Kummer S, Amon S, Manz MG, et al. Reduced CXCL4/PF4 expression as a driver of increased human hematopoietic stem and progenitor cell proliferation in polycythemia vera. Blood Cancer J. 2021;11:31.
Katoh O, Kimura A, Kuramoto A. Platelet-derived growth factor is decreased in patients with myeloproliferative disorders. Am J Hematol. 1988;27:276–80.
Starlinger P, Moll HP, Assinger A, Nemeth C, Hoetzenecker K, Gruenberger B, et al. Thrombospondin-1: a unique marker to identify in vitro platelet activation when monitoring in vivo processes. J Thromb Haemost. 2010;8:1809–19.
Acknowledgements
We thank Amgen Inc for providing Romiplostim-Nplate®; the animal facility and the OPBA of the University of Pavia for hosting the animals and support in animal protocol drawing up and the Unitech OMICs platform at the University of Milan for Orbitrap LC-MS/MS analysis. We thank Prof. Christian Di Buduo and Dr. Carolina Paula Miguel (University of Pavia) for technical assistance with confocal microscopy analysis. This paper was supported by Associazione Italiana per la Ricerca sul Cancro (AIRC MFAG 2020 24541) to AM, (AIRC IG 2016 18700) to AB; Italian Ministry of University and Research (PRIN 2017-Z5LR5Z) to AB; Italian Ministry of Health (Ricerca Finalizzata Giovani Ricercatori GR-2016-02363136) to AM and VA, Cariplo Foundation (2018-0525) to AM.
Author information
Authors and Affiliations
Contributions
DCap, FRC performed experiments, collected, and analyzed data, and wrote the manuscript. VA and MM performed experiments, collected and analyzed data. CG conceived the study and provided intellectual input. DCat, CB and AI recruited and governed the patient’s ethics, performed experiments, analyzed data, and helped to draft the manuscript. NB and AMV provided specimens from JAK2floxed/+ and JAK2V617F/+ mice. UG interpreted the data and helped write the manuscript. DT helped with histopathological analyses. AB and AM conceived the study, interpreted the data and helped write the manuscript. All authors provided input on and reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
AI is speaker honoraria from AOP Health, BMS, GSK, Incyte, Novartis and Pfizer. DC is speaker honoraria from BMS, GSK, Incyte, Novartis and Pfizer; the remaining authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: In this article the author’s name Niccolò Bartalucci was incorrectly written as Bartalucci Niccolò. The original article has been corrected.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Capitanio, D., Calledda, F.R., Abbonante, V. et al. Proteomic screening identifies PF4/Cxcl4 as a critical driver of myelofibrosis. Leukemia 38, 1971–1984 (2024). https://doi.org/10.1038/s41375-024-02354-z
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41375-024-02354-z