Abstract
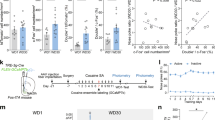
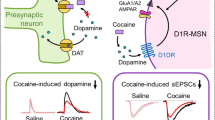
Relapse remains a major challenge to the treatment of cocaine addiction. Recent studies suggested that the trace amine-associated receptor 1 (TAAR1) could be a promising target to treat cocaine addiction and relapse; however, the underlying mechanism remains unclear. Here, we aimed to investigate the neural mechanism underlying the role of TAAR1 in the drug priming-induced reinstatement of cocaine-seeking behavior in rats, an animal model of cocaine relapse. We focused on the shell subregion of nucleus accumbens (NAc), a key brain region of the brain reward system. We found that activation of TAAR1 by systemic and intra-NAc shell administration of the selective TAAR1 agonist RO5166017 attenuated drug-induced reinstatement of cocaine-seeking and prevented drug priming-induced CaMKIIα activity in the NAc shell. Activation of TAAR1 dampened the CaMKIIα/GluR1 signaling pathway in the NAc shell and reduced AMPAR-EPSCs on the NAc slice. Microinjection of the selective TAAR1 antagonist EPPTB into the NAc shell enhanced drug-induced reinstatement as well as potentiated CaMKIIα activity in the NAc shell. Furthermore, viral-mediated expression of CaMKIIα in the NAc shell prevented the behavioral effects of TAAR1 activation. Taken together, our findings indicate that TAAR1 regulates drug-induced reinstatement of cocaine-seeking by negatively regulating CaMKIIα activity in the NAc. Our findings elucidate a novel mechanism of TAAR1 in regulating drug-induced reinstatement of cocaine-seeking and further suggests that TAAR1 is a promising target for the treatment of cocaine relapse.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Dong Y, Taylor JR, Wolf ME, Shaham Y. Circuit and synaptic plasticity mechanisms of drug relapse. J Neurosci. 2017;37:10867–76.
Kampman KM. The treatment of cocaine use disorder. Sci Adv. 2019;5:eaax1532.
Liu J, Wu R, Li JX. TAAR1 and psychostimulant addiction. Cell Mol Neurobiol. 2020;40:229–38.
Liu J, Johnson B, Wu R, Seaman R Jr., Vu J, Zhu Q, et al. TAAR1 agonists attenuate extended-access cocaine self-administration and yohimbine-induced reinstatement of cocaine-seeking. Br J Pharm. 2020;177:3403–14.
Schwartz MD, Canales JJ, Zucchi R, Espinoza S, Sukhanov I, Gainetdinov RR. Trace amine-associated receptor 1: a multimodal therapeutic target for neuropsychiatric diseases. Expert Opin Ther Targets. 2018;22:513–26.
Grandy DK. Trace amine-associated receptor 1-Family archetype or iconoclast? Pharm Ther. 2007;116:355–90.
Pei Y, Lee J, Leo D, Gainetdinov RR, Hoener MC, Canales JJ. Activation of the trace amine-associated receptor 1 prevents relapse to cocaine seeking. Neuropsychopharmacology. 2014;39:2299–308.
Thorn DA, Jing L, Qiu Y, Gancarz-Kausch AM, Galuska CM, Dietz DM, et al. Effects of the trace amine-associated receptor 1 agonist RO5263397 on abuse-related effects of cocaine in rats. Neuropsychopharmacology. 2014;39:2309–16.
Revel FG, Moreau JL, Gainetdinov RR, Bradaia A, Sotnikova TD, Mory R, et al. TAAR1 activation modulates monoaminergic neurotransmission, preventing hyperdopaminergic and hypoglutamatergic activity. Proc Natl Acad Sci USA. 2011;108:8485–90.
Revel FG, Meyer CA, Bradaia A, Jeanneau K, Calcagno E, Andre CB, et al. Brain-specific overexpression of trace amine-associated receptor 1 alters monoaminergic neurotransmission and decreases sensitivity to amphetamine. Neuropsychopharmacology. 2012;37:2580–92.
Leo D, Mus L, Espinoza S, Hoener MC, Sotnikova TD, Gainetdinov RR. Taar1-mediated modulation of presynaptic dopaminergic neurotransmission: role of D2 dopamine autoreceptors. Neuropharmacology. 2014;81:283–91.
Liu JF, Siemian JN, Seaman R Jr., Zhang Y, Li JX. Role of TAAR1 within the subregions of the mesocorticolimbic dopaminergic system in cocaine-seeking behavior. J Neurosci. 2017;37:882–92.
Espinoza S, Leo D, Sotnikova TD, Shahid M, Kaariainen TM, Gainetdinov RR. Biochemical and functional characterization of the trace amine-associated receptor 1 (TAAR1) agonist RO5263397. Front Pharm. 2018;9:645.
Asif-Malik A, Hoener MC, Canales JJ. Interaction between the trace amine-associated receptor 1 and the dopamine D2 receptor controls cocaine’s neurochemical actions. Sci Rep. 2017;7:13901.
Berry MD, Gainetdinov RR, Hoener MC, Shahid M. Pharmacology of human trace amine-associated receptors: therapeutic opportunities and challenges. Pharm Ther. 2017;180:161–80.
Harmeier A, Obermueller S, Meyer CA, Revel FG, Buchy D, Chaboz S, et al. Trace amine-associated receptor 1 activation silences GSK3beta signaling of TAAR1 and D2R heteromers. Eur Neuropsychopharmacol. 2015;25:2049–61.
Espinoza S, Salahpour A, Masri B, Sotnikova TD, Messa M, Barak LS, et al. Functional interaction between trace amine-associated receptor 1 and dopamine D2 receptor. Mol Pharm. 2011;80:416–25.
Jia W, Kawahata I, Cheng A, Fukunaga K. The role of CaMKII and ERK signaling in addiction. Int J Mol Sci. 2021;22:3189.
Anderson SM, Famous KR, Sadri-Vakili G, Kumaresan V, Schmidt HD, Bass CE, et al. CaMKII: a biochemical bridge linking accumbens dopamine and glutamate systems in cocaine seeking. Nat Neurosci. 2008;11:344–53.
Liu X, Liu Y, Zhong P, Wilkinson B, Qi J, Olsen CM, et al. CaMKII activity in the ventral tegmental area gates cocaine-induced synaptic plasticity in the nucleus accumbens. Neuropsychopharmacology. 2014;39:989–99.
Burgdorf CE, Schierberl KC, Lee AS, Fischer DK, Van Kempen TA, Mudragel V, et al. Extinction of contextual cocaine memories requires Cav1.2 within D1R-expressing cells and recruits hippocampal Cav1.2-dependent signaling mechanisms. J Neurosci. 2017;37:11894–911.
Espinoza S, Lignani G, Caffino L, Maggi S, Sukhanov I, Leo D, et al. TAAR1 modulates cortical glutamate NMDA receptor function. Neuropsychopharmacology. 2015;40:2217–27.
Liu JF, Seaman R Jr., Siemian JN, Bhimani R, Johnson B, Zhang Y, et al. Role of trace amine-associated receptor 1 in nicotine’s behavioral and neurochemical effects. Neuropsychopharmacology. 2018;43:2435–44.
Paxinos G, Watson C The rat brain in stereotaxic coordinates (Elsevier Academic, Amsterdam), 7th Edition. 2014.
Robison AJ, Vialou V, Sun HS, Labonte B, Golden SA, Dias C, et al. Fluoxetine epigenetically alters the CaMKIIalpha promoter in nucleus accumbens to regulate DeltaFosB binding and antidepressant effects. Neuropsychopharmacology. 2014;39:1178–86.
Robison AJ, Vialou V, Mazei-Robison M, Feng J, Kourrich S, Collins M, et al. Behavioral and structural responses to chronic cocaine require a feedforward loop involving DeltaFosB and calcium/calmodulin-dependent protein kinase II in the nucleus accumbens shell. J Neurosci. 2013;33:4295–307.
Yan W, Liu JF, Han Y, Zhang W, Luo YX, Xue YX, et al. Protein kinase Mzeta in medial prefrontal cortex mediates depressive-like behavior and antidepressant response. Mol Psychiatry. 2018;23:1878–91.
Chai N, Liu JF, Xue YX, Yang C, Yan W, Wang HM, et al. Delayed noradrenergic activation in the dorsal hippocampus promotes the long-term persistence of extinguished fear. Neuropsychopharmacology. 2014;39:1933–45.
Kashima DT, Grueter BA. Toll-like receptor 4 deficiency alters nucleus accumbens synaptic physiology and drug reward behavior. Proc Natl Acad Sci USA. 2017;114:8865–70.
Caprioli D, Venniro M, Zhang M, Bossert JM, Warren BL, Hope BT, et al. Role of dorsomedial striatum neuronal ensembles in incubation of methamphetamine craving after voluntary abstinence. J Neurosci. 2017;37:1014–27.
Wolf ME, Ferrario CR. AMPA receptor plasticity in the nucleus accumbens after repeated exposure to cocaine. Neurosci Biobehav Rev. 2010;35:185–211.
Soares-Cunha C, de Vasconcelos NAP, Coimbra B, Domingues AV, Silva JM, Loureiro-Campos E, et al. Nucleus accumbens medium spiny neurons subtypes signal both reward and aversion. Mol Psychiatry. 2020;25:3241–55.
Bradaia A, Trube G, Stalder H, Norcross RD, Ozmen L, Wettstein JG, et al. The selective antagonist EPPTB reveals TAAR1-mediated regulatory mechanisms in dopaminergic neurons of the mesolimbic system. Proc Natl Acad Sci USA. 2009;106:20081–6.
Zhukov IS, Kubarskaya LG, Tissen IY, Kozlova AA, Dagayev SG, Kashuro VA, et al. Minimal age-related alterations in behavioral and hematological parameters in trace amine-associated receptor 1 (TAAR1) knockout mice. Cell Mol Neurobiol. 2020;40:273–82.
Pei Y, Asif-Malik A, Canales JJ. Trace amines and the trace amine-associated receptor 1: pharmacology, neurochemistry, and clinical implications. Front Neurosci. 2016;10:148.
Revel FG, Moreau JL, Pouzet B, Mory R, Bradaia A, Buchy D, et al. A new perspective for schizophrenia: TAAR1 agonists reveal antipsychotic- and antidepressant-like activity, improve cognition and control body weight. Mol Psychiatry. 2013;18:543–56.
Revel FG, Moreau JL, Gainetdinov RR, Ferragud A, Velazquez-Sanchez C, Sotnikova TD, et al. Trace amine-associated receptor 1 partial agonism reveals novel paradigm for neuropsychiatric therapeutics. Biol Psychiatry. 2012;72:934–42.
Miller GM. The emerging role of trace amine-associated receptor 1 in the functional regulation of monoamine transporters and dopaminergic activity. J Neurochem. 2011;116:164–76.
Underhill SM, Hullihen PD, Chen J, Fenollar-Ferrer C, Rizzo MA, Ingram SL, et al. Amphetamines signal through intracellular TAAR1 receptors coupled to Galpha13 and GalphaS in discrete subcellular domains. Mol Psychiatry. 2021;26:1208–23.
Vinade L, Dosemeci A. Regulation of the phosphorylation state of the AMPA receptor GluR1 subunit in the postsynaptic density. Cell Mol Neurobiol. 2000;20:451–63.
Liu Y, Sun QA, Chen Q, Lee TH, Huang Y, Wetsel WC, et al. Targeting inhibition of GluR1 Ser845 phosphorylation with an RNA aptamer that blocks AMPA receptor trafficking. J Neurochem. 2009;108:147–57.
Park JS, Voitenko N, Petralia RS, Guan X, Xu JT, Steinberg JP, et al. Persistent inflammation induces GluR2 internalization via NMDA receptor-triggered PKC activation in dorsal horn neurons. J Neurosci. 2009;29:3206–19.
Rutigliano G, Accorroni A, Zucchi R. The case for TAAR1 as a modulator of central nervous system function. Front Pharm. 2017;8:987.
Lindemann L, Meyer CA, Jeanneau K, Bradaia A, Ozmen L, Bluethmann H, et al. Trace amine-associated receptor 1 modulates dopaminergic activity. J Pharm Exp Ther. 2008;324:948–56.
Pei Y, Mortas P, Hoener MC, Canales JJ. Selective activation of the trace amine-associated receptor 1 decreases cocaine’s reinforcing efficacy and prevents cocaine-induced changes in brain reward thresholds. Prog Neuropsychopharmacol Biol Psychiatry. 2015;63:70–5.
Sukhanov I, Espinoza S, Yakovlev DS, Hoener MC, Sotnikova TD, Gainetdinov RR. TAAR1-dependent effects of apomorphine in mice. Int J Neuropsychopharmacol. 2014;17:1683–93.
Pitts MS, McShane JN, Hoener MC, Christian SL, Berry MD. TAAR1 levels and sub-cellular distribution are cell line but not breast cancer subtype specific. Histochem Cell Biol. 2019;152:155–66.
Sukhanov I, Dorofeikova M, Dolgorukova A, Dorotenko A, Gainetdinov RR. Trace amine-associated receptor 1 modulates the locomotor and sensitization effects of nicotine. Front Pharm. 2018;9:329.
Liu J, Seaman R Jr., Johnson B, Wu R, Vu J, Tian J, et al. Activation of trace amine-associated receptor 1 selectively attenuates the reinforcing effects of morphine. Br J Pharm. 2021;178:933–45.
Acknowledgements
We thank Dr. Marius C. Hoener at F. Hoffmann-La Roche Ltd (Basel, Switzerland) for providing with us the breeding pairs of TAAR1 KO rats. This work was supported by the National Institutes of Health National Institute on Drug Abuse (Grants R21DA040777 and R01DA047967 to J-X.L.). Kevin was supported by T32GM108554 (Training in Perioperative Science). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Contributions
JL and J-XL designed the study; JL, RW, RS, BJ, KM, JV, and YH conducted the experiments; YZ provided the TAAR1 agonists; AR and RN provided the viruses used in this study; JL, J-XL, BG, and DM prepared the manuscript. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Liu, J., Wu, R., Seaman, R. et al. TAAR1 regulates drug-induced reinstatement of cocaine-seeking via negatively modulating CaMKIIα activity in the NAc. Mol Psychiatry 27, 2136–2145 (2022). https://doi.org/10.1038/s41380-022-01448-3
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41380-022-01448-3
This article is cited by
-
Selective TAAR1 agonists induce conditioned taste aversion
Psychopharmacology (2022)