Abstract
Sub-optimal response in schizophrenia is frequent, warranting augmentation strategies over treatment-as-usual (TAU). We assessed nutraceuticals/phytoceutical augmentation strategies via network meta-analysis. Randomized controlled trials in schizophrenia/schizoaffective disorder were identified via the following databases: PubMed, MEDLINE, EMBASE, Scopus, PsycINFO, CENTRAL, and ClinicalTrials.gov. Change (Standardized Mean Difference = SMD) in total symptomatology and acceptability (Risk Ratio = RR) were co-primary outcomes. Secondary outcomes were positive, negative, cognitive, and depressive symptom changes, general psychopathology, tolerability, and response rates. We conducted subset analyses by disease phase and sensitivity analyses by risk of bias and assessed global/local inconsistency, publication bias, risk of bias, and confidence in the evidence. The systematic review included 49 records documenting 50 studies (n = 2384) documenting 22 interventions. Citicoline (SMD =−1.05,95%CI = –1.85; −0.24), L-lysine (SMD = –1.04,95%CI = –1.84; −0.25), N-acetylcysteine (SMD = –0.87, 95%CI = –1.27; −0.47) and sarcosine (SMD = –0.5,95%CI = –0.87–0.13) outperformed placebo for total symptomatology. High heterogeneity (tau2 = 0.10, I2 = 55.9%) and global inconsistency (Q = 40.79, df = 18, p = 0.002) emerged without publication bias (Egger’s test, p = 0.42). Sarcosine improved negative symptoms (SMD = –0.65, 95%CI = –1.10; −0.19). N-acetylcysteine improved negative symptoms (SMD = –0.90, 95%CI = –1.42; −0.39)/general psychopathology (SMD = –0.76, 95%CI = –1.39; −0.13). No compound improved total symptomatology within acute phase studies (k = 7, n = 422). Sarcosine (SMD = –1.26,95%CI = –1.91; −0.60), citicoline (SMD = –1.05,95%CI = –1.65;-0.44), and N-acetylcysteine (SMD = –0.55,95%CI = –0.92,−0.19) outperformed placebo augmentation in clinically stable participants. Sensitivity analyses removing high-risk-of-bias studies confirmed overall findings in all phases and clinically stable samples. In contrast, the acute phase analysis restricted to low risk-of-bias studies showed a superior effect vs. placebo for N-acetylcysteine (SMD = −1.10, 95%CI = –1.75,−0.45), L-lysine (SMD = –1.05,95%CI = –1.55, −0.19), omega-3 fatty acids (SMD = –0.83,95%CI = –1.31, −0.34) and withania somnifera (SMD = –0.71,95%CI = –1.21,−0.22). Citicoline (SMD = –1.05,95%CI = –1.86,−0.23), L-lysine (SMD = –1.04,95%CI = –1.84,−0.24), N-acetylcysteine (SMD = –0.89,95%CI = –1.35,−0.43) and sarcosine (SMD = –0.61,95%CI = –1.02,−0.21) outperformed placebo augmentation of TAU (“any phase”). Drop-out due to any cause or adverse events did not differ between nutraceutical/phytoceutical vs. placebo+TAU. Sarcosine, citicoline, and N-acetylcysteine are promising augmentation interventions in stable patients with schizophrenia, yet the quality of evidence is low to very low. Further high-quality trials in acute phases/specific outcomes/difficult-to-treat schizophrenia are warranted.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
With the publication of this article, the entire dataset will be available to the final reader upon request. All authors had access to the data and were responsible for the decision to submit it for publication.
References
GBD 2019 MDC. Global, regional, and national burden of 12 mental disorders in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Psychiatry. 2022;9:137–50.
Solmi M SG, Mavridis D, Correll CU, Dragioti E, Guimond S, et al. Incidence, prevalence, and global burden of schizophrenia—data, with critical appraisal, from the Global Burden of Disease (GBD) 2019. Mol Psychiatry. 2023.
Keepers GA, Fochtmann LJ, Anzia JM, Benjamin S, Lyness JM, Mojtabai R, et al. The American Psychiatric Association practice guideline for the treatment of patients with schizophrenia. Am J Psychiatry. 2020;177:868–72.
Correll CU, Howes OD. Treatment-resistant schizophrenia: definition, predictors, and therapy options. J Clin Psychiatry. 2021; 82:MY20096AH1C.
Diniz E, Fonseca L, Rocha D, Trevizol A, Cerqueira R, Ortiz B, et al. Treatment resistance in schizophrenia: a meta-analysis of prevalence and correlates. Braz J Psychiatry. 2023;45:448–58.
Correll CU, Solmi M, Cortese S, Fava M, Højlund M, Kraemer HC, et al. The future of psychopharmacology: a critical appraisal of ongoing phase 2/3 trials, and of some current trends aiming to de‐risk trial programmes of novel agents. World Psychiatry. 2023;22:48–74.
Berk M, Marx W, Nierenberg AA. The aggregation of marginal gains: a pragmatic philosophy of clinical care in psychiatry. World Psychiatry. 2024;23:291–292.
Correll CU, Rubio JM, Inczedy-Farkas G, Birnbaum ML, Kane JM, Leucht S. Efficacy of 42 pharmacologic cotreatment strategies added to antipsychotic monotherapy in schizophrenia: systematic overview and quality appraisal of the meta-analytic evidence. JAMA Psychiatry. 2017;74:675–84.
Seetharaman M, Krishnan G, Schneider RH. The future of medicine: frontiers in integrative health and medicine. Medicina. 2021;57:1303.
Tsiaka T, Kritsi E, Tsiantas K, Christodoulou P, Sinanoglou VJ, Zoumpoulakis P. Design and development of novel nutraceuticals: current trends and methodologies. Nutraceuticals. 2022;2:71–90.
Sarris J, Ravindran A, Yatham LN, Marx W, Rucklidge JJ, McIntyre RS et al. Clinician guidelines for the treatment of psychiatric disorders with nutraceuticals and phytoceuticals: The World Federation of Societies of Biological Psychiatry (WFSBP) and Canadian Network for Mood and Anxiety Treatments (CANMAT) Taskforce. World J Biol Psychiatry. 2022:1–32.
DeFelice SL. The nutraceutical revolution: its impact on food industry R&D. Trends Food Sci Technol. 1995;6:59–61.
Lee SY, Shin YW, Hahm KB. Phytoceuticals: mighty but ignored weapons against Helicobacter pylori infection. J Dig Dis. 2008;9:129–39.
Firth J, Teasdale SB, Allott K, Siskind D, Marx W, Cotter J, et al. The efficacy and safety of nutrient supplements in the treatment of mental disorders: a meta-review of meta-analyses of randomized controlled trials. World Psychiatry. 2019;18:308–24.
Sarris J, Marx W, Ashton MM, Ng CH, Galvao-Coelho N, Ayati Z, et al. Plant-based medicines (Phytoceuticals) in the treatment of psychiatric disorders: a meta-review of meta-analyses of randomized controlled trials: Les médicaments à base de plantes (phytoceutiques) dans le traitement des troubles psychiatriques: une méta-revue des méta-analyses d’essais randomisés contrôlés. Can J Psychiatry. 2021;66:849–62.
Xu X, Shao G, Zhang X, Hu Y, Huang J, Su Y, et al. The efficacy of nutritional supplements for the adjunctive treatment of schizophrenia in adults: A systematic review and network meta-analysis. Psychiatry Res. 2022;311:114500.
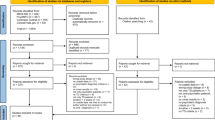
Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162:777–84.
Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898.
Nikolakopoulou A, Higgins JP, Papakonstantinou T, Chaimani A, Del Giovane C, Egger M, et al. CINeMA: an approach for assessing confidence in the results of a network meta-analysis. PLoS Med. 2020;17:e1003082.
Higgins JPT TJ, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane handbook for systematic reviews of interventions version 6.3 (updated February 2022), 2022.
Solmi M, Wade TD, Byrne S, Del Giovane C, Fairburn CG, Ostinelli EG, et al. Comparative efficacy and acceptability of psychological interventions for the treatment of adult outpatients with anorexia nervosa: a systematic review and network meta-analysis. Lancet Psychiatry. 2021;8:215–24.
Levine J, Stahl Z, Sela BA, Ruderman V, Shumaico O, Babushkin I, et al. Homocysteine-reducing strategies improve symptoms in chronic schizophrenic patients with hyperhomocysteinemia. Biol Psychiatry. 2006;60:265–9.
Hosseininasab M, Zarghami M, Mazhari S, Salehifar E, Moosazadeh M, Fariborzifar A, et al. Nanocurcumin as an add-on to antipsychotic drugs for treatment of negative symptoms in patients with chronic schizophrenia: a randomized, double-blind, placebo-controlled study. J Clin Psychopharmacol. 2021;41:25–30.
Miodownik C, Lerner V, Kudkaeva N, Lerner PP, Pashinian A, Bersudsky Y, et al. Curcumin as add-on to antipsychotic treatment in patients with chronic schizophrenia: A randomized, double-blind, placebo-controlled study. Clin Neuropharmacol. 2019;42:117–22.
Roffman JL, Lamberti JS, Achtyes E, Macklin EA, Galendez GC, Raeke LH, et al. Randomized multicenter investigation of folate plus vitamin B12 supplementation in schizophrenia. JAMA Psychiatry. 2013;70:481–9.
Roffman JL, Petruzzi LJ, Tanner AS, Brown HE, Eryilmaz H, Ho NF, et al. Biochemical, physiological and clinical effects of l-methylfolate in schizophrenia: a randomized controlled trial. Mol Psychiatry. 2018;23:316–22.
Berk M, Copolov D, Dean O, Lu K, Jeavons S, Schapkaitz I, et al. N-acetyl cysteine as a glutathione precursor for schizophrenia-a double-blind, randomized, placebo-controlled trial. Biol Psychiatry. 2008;64:361–8.
Krivoy A, Onn R, Vilner Y, Hochman E, Weizman S, Paz A, et al. Vitamin D supplementation in chronic schizophrenia patients treated with clozapine: a randomized, double-blind, placebo-controlled clinical trial. EBioMedicine. 2017;26:138–45.
Sepehrmanesh Z, Heidary M, Akasheh N, Akbari H, Heidary M. Therapeutic effect of adjunctive N-acetyl cysteine (NAC) on symptoms of chronic schizophrenia: a double-blind, randomized clinical trial. Prog Neuro-Psychopharmacol Biol Psychiatry. 2018;82:289–96.
Sheikhmoonesi F, Zarghami M, Mamashli S, Charati JY, Hamzehpour R, Fattahi S, et al. Effectiveness of vitamin D supplement therapy in chronic stable schizophrenic male patients: a randomized controlled trial. Iran J Pharm Res. 2016;15:941–50.
Farokhnia M, Azarkolah A, Adinehfar F, Khodaie-Ardakani MR, Hosseini SM, Yekehtaz H, et al. N-acetylcysteine as an adjunct to risperidone for treatment of negative symptoms in patients with chronic schizophrenia: a randomized, double-blind, placebo-controlled study. Clin Neuropharmacol. 2013;36:185–192.
Breier A, Liffick E, Hummer TA, Vohs JL, Yang Z, Mehdiyoun NF, et al. Effects of 12-month, double-blind N-acetyl cysteine on symptoms, cognition and brain morphology in early phase schizophrenia spectrum disorders. Schizophr Res. 2018;199:395–402.
Qiao Y, Liu CP, Han HQ, Liu FJ, Shao Y, Xie B. No impact of omega-3 fatty acid supplementation on symptoms or hostility among patients with schizophrenia. Front Psychiatry. 2020;11:312.
Peet M, Brind J, Ramchand CN, Shah S, Vankar GK. Two double-blind placebo-controlled pilot studies of eicosapentaenoic acid in the treatment of schizophrenia. Research. 2001;49:243–51.
Peet M, Horrobin DF. A dose-ranging exploratory study of the effects of ethyl-eicosapentaenoate in patients with persistent schizophrenic symptoms. J Psychiatr Res. 2002;36:7–18.
Pawełczyk T, Grancow-Grabka M, Kotlicka-Antczak M, Trafalska E, Pawełczyk A. A randomized controlled study of the efficacy of six-month supplementation with concentrated fish oil rich in omega-3 polyunsaturated fatty acids in first episode schizophrenia. J Psychiatr Res. 2016;73:34–44.
Jamilian H, Ghaderi A. The effects of probiotic and selenium co-supplementation on clinical and metabolic scales in chronic schizophrenia: a randomized, double-blind, placebo-controlled trial. Biol Trace Elem Res. 2021;199:4430–8.
Jamilian H, Solhi H, Jamilian M. Randomized, placebo-controlled clinical trial of omega-3 as supplemental treatment in schizophrenia. Glob J Health Sci. 2014;6:103–8.
Emsley R, Chiliza B, Asmal L, du Plessis S, Phahladira L, van Niekerk E, et al. A randomized, controlled trial of omega-3 fatty acids plus an antioxidant for relapse prevention after antipsychotic discontinuation in first-episode schizophrenia. Schizophr Res. 2014;158:230–5.
Emsley R, Myburgh C, Oosthuizen P, Van Rensburg SJ. Randomized, placebo-controlled study of ethyl-eicosapentaenoic acid as supplemental treatment in schizophrenia. Am J Psychiatry. 2002;159:1596–8.
Tang W, Wang Y, Xu F, Fan W, Zhang Y, Fan K, et al. Omega-3 fatty acids ameliorate cognitive dysfunction in schizophrenia patients with metabolic syndrome. Brain Behav Immun. 2020;88:529–34.
Ritsner MS, Miodownik C, Ratner Y, Shleifer T, Mar I, Pintov L, et al. L-theanine relieves positive, activation, and anxiety symptoms in patients with schizophrenia and schizoaffective disorder: An 8-week, randomized, double-blind, placebo-controlled, 2-center study. J Clin Psychiatry. 2011;72:34–42.
Ghaderi A, Banafshe HR, Mirhosseini N, Moradi M, Karimi MA, Mehrzad F, et al. Clinical and metabolic response to vitamin D plus probiotic in schizophrenia patients. BMC Psychiatry. 2019;19:77.
Chengappa KNR, Brar JS, Gannon JM, Schlicht PJ. Adjunctive use of a standardized extract of withania somnifera (ashwagandha) to treat symptom exacerbation in schizophrenia: a randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2018;79:17m11826.
Chen EY, Hui CL. HT1001, a proprietary North American ginseng extract, improves working memory in schizophrenia: a double-blind, placebo-controlled study. Phytother Resem. 2012;26:1166–1172.
Atmaca M, Tezcan E, Kuloglu M, Ustundag B, Kirtas O. The effect of extract of ginkgo biloba addition to olanzapine on therapeutic effect and antioxidant enzyme levels in patients with schizophrenia. Psychiatry Clin Neurosci. 2005;59:652–6.
Zhang XY, Zhou DF, Su JM, Zhang PY. The effect of extract of ginkgo biloba added to haloperidol on superoxide dismutase in inpatients with chronic schizophrenia. J Clin Psychopharmacol. 2001;21:85–88.
Doruk A, Uzun Ö, Özşahin A. A placebo-controlled study of extract of ginkgo biloba added to clozapine in patients with treatment-resistant schizophrenia. Int Clin Psychopharmacol. 2008;23:223–7.
Miyaoka T, Furuya M, Horiguchi J, Wake R, Hashioka S, Tohyama M, et al. Efficacy and safety of yokukansan in treatment-resistant schizophrenia: a randomized, double-blind, placebo-controlled trial (a Positive and Negative Syndrome Scale, five-factor analysis). Psychopharmacology. 2015;232:155–164.
Dickerson F, Origoni A, Katsafanas E, Squire A, Newman T, Fahey J, et al. Randomized controlled trial of an adjunctive sulforaphane nutraceutical in schizophrenia. Schizophr Res. 2021;231:142–144.
Xiao SF, Xue HB, Li X, Chen C, Li GJ, Yuan CM, et al. A double-blind, placebo-controlled study of traditional Chinese medicine sarsasapogenin added to risperidone in patients with negative symptoms dominated schizophrenia. Neurosci Bull. 2011;27:258–68.
Lane HY, Chang YC, Liu YC, Chiu CC, Tsai GE. Sarcosine or D-serine add-on treatment for acute exacerbation of schizophrenia: a randomized, double-blind, placebo-controlled study. Arch Gen Psychiatry. 2005;62:1196–204.
Lane HY, Huang CL, Wu PL, Liu YC, Chang YC, Lin PY, et al. Glycine transporter I Inhibitor, N-methylglycine (Sarcosine), added to clozapine for the treatment of schizophrenia. Biol Psychiatry. 2006;60:645–9.
Lane HY, Lin CH, Huang YJ, Liao CH, Chang YC, Tsai GE. A randomized, double-blind, placebo-controlled comparison study of sarcosine (N-methylglycine) and d-serine add-on treatment for schizophrenia. Int J Neuropsychopharmacol. 2010;13:451–60.
Tsai G, Lane HY, Yang P, Chong MY, Lange N. Glycine transporter I inhibitor, N-Methylglycine (sarcosine), added to antipsychotics for the treatment of schizophrenia. Biol Psychiatry. 2004;55:452–6.
Tsai GE, Yang P, Chung LC, Tsai IC, Tsai CW, Coyle JTD-. serine added to clozapine for the treatment of schizophrenia. Am J Psychiatry. 1999;156:1822–5.
Zeinoddini A, Ahadi M, Farokhnia M, Rezaei F, Tabrizi M, Akhondzadeh S. L-lysine as an adjunct to risperidone in patients with chronic schizophrenia: a double-blind, placebo-controlled, randomized trial. J Psychiatr Res. 2014;59:125–31.
Lin CY, Liang SY, Chang YC, Ting SY, Kao CL, Wu YH, et al. Adjunctive sarcosine plus benzoate improved cognitive function in chronic schizophrenia patients with constant clinical symptoms: a randomised, double-blind, placebo-controlled trial. World J Biol Psychiatry. 2017;18:357–68.
Strzelecki D, Urban-Kowalczyk M, Wysokiński A. Serum levels of interleukin 6 in schizophrenic patients during treatment augmentation with sarcosine (results of the PULSAR study). Hum Psychopharmacol. 2018;33:e2652.
Ghajar A, Gholamian F, Tabatabei-Motlagh M, Afarideh M, Rezaei F, Ghazizadeh-Hashemi M et al. Citicoline (CDP-choline) add-on therapy to risperidone for treatment of negative symptoms in patients with stable schizophrenia: a double-blind, randomized placebo-controlled trial. Hum. Psychopharmacol. 2018;33:e2662.
Turner A, Baker A, Dean OM, Walker AJ, Dodd S, Cotton SM, et al. Adjunctive Garcinia mangostana Linn. (Mangosteen) Pericarp for schizophrenia: a 24-week double-blind, randomized, placebo controlled efficacy trial: Péricarpe d’appoint Garcinia mangostana Linn (mangoustan) pour la schizophrénie: un essai d’efficacité de 24 semaines, à double insu, randomisé et contrôlé par placebo. Can J Psychiatry. 2021;66:354–66.
Serrita J, Ralevski E, Yoon G, Petrakis I. A pilot randomized, placebo-controlled trial of glycine for treatment of schizophrenia and alcohol dependence. J Dual Diagn. 2019;15:46–55.
Xiao S, Xue H, Li G, Yuan C, Li X, Chen C, et al. Therapeutic effects of cerebrolysin added to risperidone in patients with schizophrenia dominated by negative symptoms. Aust NZ J psychiatry. 2012;46:153–60.
D’Souza DC, Radhakrishnan R, Perry E, Bhakta SG, Singh N, Pittman B, et al. Feasibility, safety and efficacy of the combination of D-serine and computerized cognitive retraining in schizophrenia: an international collaborative pilot study. Schizophr. Bull. 2013;39:S326.
Evins AE, Fitzgerald SM, Wine L, Rosselli R, Goff DC. Placebo-controlled trial of glycine added to clozapine in schizophrenia. Am J Psychiatry. 2000;157:826–8.
Buchanan RW, Javitt DC, Marder SR, Schooler NR, Gold JM, McMahon RP, et al. The Cognitive and Negative Symptoms in Schizophrenia Trial (CONSIST): the efficacy of glutamatergic agents for negative symptoms and cognitive impairments. Am J Psychiatry. 2007;164:1593–602.
Dakhale GN, Khanzode SD, Khanzode SS, Saoji A. Supplementation of vitamin C with atypical antipsychotics reduces oxidative stress and improves the outcome of schizophrenia. Psychopharmacology. 2005;182:494–8.
Heresco-Levy U, Javitt DC, Ebstein R, Vass A, Lichtenberg P, Bar G, et al. D-serine efficacy as add-on pharmacotherapy to risperidone and olanzapine for treatment-refractory schizophrenia. Biol Psychiatry. 2005;57:577–85.
Fenton WS, Dickerson F, Boronow J, Hibbeln JR, Knable M. A placebo-controlled trial of omega-3 fatty acid (ethyl eicosapentaenoic acid) supplementation for residual symptoms and cognitive impairment in schizophrenia. Am J Psychiatry. 2001;158:2071–4.
Hill M, Shannahan K, Jasinski S, Macklin EA, Raeke L, Roffman JL, et al. Folate supplementation in schizophrenia: a possible role for MTHFR genotype. Schizophr Res. 2011;127:41–45.
Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–76.
Andreasen NC. Scale for the assessment of positive symptoms. Psychiatr Psychobiol. University of Iowa, Iowa City. 1984.
Andreasen NC. Scale for the assessment of negative symptoms (SANS). University of Iowa Iowa City. 1981.
Guy W. Clinical global impression. Assessment manual for psychopharmacology 1976: 217–22.
Nasyrova RF, Ivashchenko DV, Ivanov MV, Neznanov NG. Role of nitric oxide and related molecules in schizophrenia pathogenesis: biochemical, genetic and clinical aspects. Front Physiol. 2015;6:139.
Ng F, Berk M, Dean O, Bush AI. Oxidative stress in psychiatric disorders: evidence base and therapeutic implications. Int J Neuropsychopharmacol. 2008;11:851–76.
Matsuzawa D, Obata T, Shirayama Y, Nonaka H, Kanazawa Y, Yoshitome E, et al. Negative correlation between brain glutathione level and negative symptoms in schizophrenia: a 3T 1H-MRS study. PloS One. 2008;3:e1944.
Müller N, Weidinger E, Leitner B, Schwarz MJ. The role of inflammation in schizophrenia. Front Neurosci. 2015;9:372.
Marx W, McGuinness AJ, Rocks T, Ruusunen A, Cleminson J, Walker AJ, et al. The kynurenine pathway in major depressive disorder, bipolar disorder, and schizophrenia: a meta-analysis of 101 studies. Mol Psychiatry. 2021;26:4158–78.
McCutcheon RA, Krystal JH, Howes OD. Dopamine and glutamate in schizophrenia: biology, symptoms and treatment. World Psychiatry. 2020;19:15–33.
Cole GM, Teter B, Frautschy SA. Neuroprotective effects of curcumin. Adv Exp Med Biol. 2007;595:197–212.
Mallah K, Couch C, Borucki DM, Toutonji A, Alshareef M, Tomlinson S. Anti-inflammatory and neuroprotective agents in clinical trials for CNS disease and injury: where do we go from here? Front Immunol. 2020;11:2021.
Conant R, Schauss AG. Therapeutic applications of citicoline for stroke and cognitive dysfunction in the elderly: a review of the literature. Altern Med Rev. 2004;9:17–31.
Skvarc DR, Dean OM, Byrne LK, Gray L, Lane S, Lewis M, et al. The effect of N-acetylcysteine (NAC) on human cognition—a systematic review. Neurosci Biobehav Rev. 2017;78:44–56.
Kumar A. NMDA receptor function during senescence: implication on cognitive performance. Front Neurosci. 2015;9:473.
Klauser P, Xin L, Fournier M, Griffa A, Cleusix M, Jenni R, et al. N-acetylcysteine add-on treatment leads to an improvement of fornix white matter integrity in early psychosis: a double-blind randomized placebo-controlled trial. Transl Psychiatry. 2018;8:220.
Mullier E, Roine T, Griffa A, Xin L, Baumann PS, Klauser P, et al. N-acetyl-cysteine supplementation improves functional connectivity within the cingulate cortex in early psychosis: a pilot study. Int J Neuropsychopharmacol. 2019;22:478–87.
Fond G., Mallet J., Urbach M., Eriksen Benros M., Berk M., et al. Adjunctive agents to antipsychotics in schizophrenia: a systematic umbrella review and recommendations for amino acids, hormonal therapies and anti-inflammatory drugs. BMJ Ment Health. 2023; e300771.
Tanas A, Tozlu Ö, Gezmiş T, Hacimüftüoğlu A, Abd El-Aty AM, Ceylan O, et al. In vitro and in vivo neuroprotective effects of sarcosine. Biomed Res Int. 2022;2022:5467498.
de Bartolomeis A, Vellucci L, Austin MC, De Simone G, Barone A. Rational and translational implications of D-amino acids for treatment-resistant schizophrenia: from neurobiology to the clinics. Biomolecules. 2022;12:909.
Marchi M, Galli G, Magarini FM, Mattei G, Galeazzi GM. Sarcosine as an add-on treatment to antipsychotic medication for people with schizophrenia: a systematic review and meta-analysis of randomized controlled trials. Expert Opin Drug Metab Toxicol. 2021;17:483–93.
Mitra S, Natarajan R, Ziedonis D, Fan X. Antioxidant and anti-inflammatory nutrient status, supplementation, and mechanisms in patients with schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2017;78:1–11.
van der Burg KP, Cribb L, Firth J, Karmacoska D, Sarris J. Nutrient and genetic biomarkers of nutraceutical treatment response in mood and psychotic disorders: a systematic review. Nutr Neurosci. 2021;24:279–95.
Santini A, Cammarata SM, Capone G, Ianaro A, Tenore GC, Pani L, et al. Nutraceuticals: opening the debate for a regulatory framework. Br J Clin Pharmacol. 2018;84:659–72.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
Funding
MB is supported by an NHMRC Senior Principal Research Fellowship and Leadership 3 Investigator grant (1156072 and 2017131). WM is currently funded by an NHMRC Investigator Grant (#2008971)
Author information
Authors and Affiliations
Contributions
MF and MB conceived the study, which MF and CaC coordinated. CaC, NS, MDP, MS, and CUC assisted in the statistical analysis and critical interpretation of the results. MF and CaC wrote the manuscript, which all authors read and approved.
Corresponding author
Ethics declarations
Competing interests
MF received honoraria for his speaker activity from the American Society of Clinical Psychopharmacology (ASCP) and served as a consultant for Angelini, Otsuka, Lundbeck, Sanofi-Aventis, and Boehringer Ingelheim. WM has previously received university grants/fellowships from La Trobe University, Deakin University, University of Queensland, and Bond University. WM has received funding and/or has attended events funded by Cobram Estate Pty. Ltd and Bega Dairy and Drinks Pty Ltd. WM has received travel funding from the Nutrition Society of Australia. WM has received consultancy funding from Nutrition Research Australia and ParachuteBH. MS has received honoraria/has been a consultant for AbbVie, Angelini, Lundbeck, and Otsuka. VBM received honoraria from Angelini, unrelated to the present work. CUC has been a consultant and/or advisor to or has received honoraria from: AbbVie, Acadia, Adock Ingram, Alkermes, Allergan, Angelini, Aristo, Biogen, Boehringer-Ingelheim, Bristol-Meyers Squibb, Car dio Diagnostics, Cerevel, CNX Therapeutics, Compass Pathways, Darnitsa, Delpor, Denovo, Gedeon Richter, Hikma, Holmusk, IntraCellular Therapies, Jamjoom Pharma, Janssen/J&J, Karuna, LB Pharma, Lundbeck, MedAvante-ProPhase, MedInCell, Merck, Mindpax, Mitsubishi Tanabe Pharma, Mylan, Neurocrine, Neurelis, Newron, Noven, Novo Nordisk, Otsuka, Pharmabrain, PPD Biotech, Recordati, Relmada, Reviva, Rovi, Sage, Seqirus, SK Life Science, Sumitomo Pharma America, Sunovion, Sun Pharma, Supernus, Tabuk, Takeda, Teva, Tolmar, Vertex, and Viatris. He provided expert testimony for Janssen and Otsuka. He served on a Data Safety Monitoring Board for Compass Pathways, Denovo, Lundbeck, Relmada, Reviva, Rovi, Supernus, and Teva. He has received grant support from Janssen and Takeda. He received royalties from UpToDate and is also a stock option holder of Cardio Diagnostics, Kuleon Biosciences, LB Pharma, Mindpax, and Quantic. All other authors declare no conflicts of interest relevant to this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fornaro, M., Caiazza, C., Billeci, M. et al. Nutraceuticals and phytoceuticals in the treatment of schizophrenia: a systematic review and network meta-analysis “Nutra NMA SCZ”. Mol Psychiatry 30, 168–187 (2025). https://doi.org/10.1038/s41380-024-02645-y
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41380-024-02645-y
This article is cited by
-
Targeted metabolomics study on peripheral blood neurotransmitters in early-onset schizophrenia
Journal of Translational Medicine (2025)
-
Efficacy and safety of sulforaphane in schizophrenia: a systematic review and meta-analysis of randomized controlled trials
BMC Psychiatry (2025)
-
Nahrungsergänzungsmittel und Phytozeutika bei psychischen Störungen
DNP – Die Neurologie & Psychiatrie (2025)
-
Modulation of glycine transporters as a novel therapeutic strategy in neuropsychiatry
Psychopharmacology (2025)