Abstract
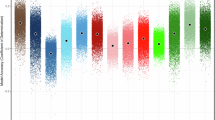
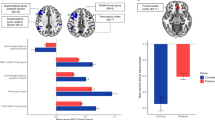
Objective markers of pathophysiological processes underlying lifetime depression and mania/hypomania risk can provide biologically informed targets for novel interventions to help prevent the onset of affective disorders in individuals with subsyndromal symptoms. Greater activity within and functional connectivity (FC) between the central executive network (CEN), supporting emotional regulation (ER) subcomponent processes such as working memory (WM), the default mode network (DMN), supporting self-related information processing, and the salience network (SN), is thought to interfere with cognitive functioning and predispose to depressive disorders. Using an emotional n-back paradigm designed to examine WM and ER capacity, we examined in young adults: (1) relationships among activity and FC in these networks and lifetime depression and mania/hypomania risk; (2) the extent to which these relationships were specific to lifetime depression risk versus lifetime mania/hypomania risk; (3) whether findings in a first, Discovery sample n = 101, 63 female, age = 23.85 (2.9) could be replicated in a two independent Test samples of young adults: Test sample 1: n = 90, 60 female, age = 21.7 (2.0); Test sample 2: n = 96, 65 female, age = 21.6 (2.1). The Mood Spectrum Self-Report (MOODS-SR-L) assessed lifetime mania/hypomania risk and depression risk. We showed significant clusters of activity to each contrast in similar locations in the anatomic mask in each Test sample as in the Discovery sample, and, using extracted mean BOLD signal from these clusters as IVs, we showed similar patterns of IV-DV relationships in each Test sample as in the Discovery sample. Specifically, in the Discovery sample, greater DMN activity during WM was associated with greater lifetime depression risk. This finding was specific to depression and replicated in both independent samples (all ps<0.05 qFDR). Greater CEN activity during ER was associated with increased lifetime depression risk and lifetime mania/hypomania risk in all three samples (all ps< 0.05 qFDR). These replicated findings provide promising objective, neural markers to better identify, and guide and monitor early interventions for, depression and mania/hypomania risk in young adults.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The dataset is available per request. Please request access by contacting the corresponding author Yvette Afriyie-Agyemang.
References
Simon GE. Social and economic burden of mood disorders. Biol Psychiatry. 2003;54:208–15.
Lima IMM, Peckham AD, Johnson SL. Cognitive deficits in bipolar disorders: Implications for emotion. Clin Psychol Rev. 2018;59:126–36.
Merikangas KR, Jin R, He JP, Kessler RC, Lee S, Sampson NA, et al. Prevalence and correlates of bipolar spectrum disorder in the world mental health survey initiative. Arch Gen Psychiatry. 2011;68:241–51.
Korgaonkar MS, Erlinger M, Breukelaar IA, Boyce P, Hazell P, Antees C, et al. Amygdala activation and connectivity to emotional processing distinguishes asymptomatic patients with bipolar disorders and unipolar depression. Biol Psychiatry Cogn Neurosci Neuroimaging. 2019;4:361–70.
Diamond A. Executive functions. Annu Rev Psychol. 2013;64:135–68.
Nikolin S, Tan YY, Schwaab A, Moffa A, Loo CK, Martin D. An investigation of working memory deficits in depression using the n-back task: A systematic review and meta-analysis. J Affect Disord. 2021;284:1–8.
Snyder HR. Major depressive disorder is associated with broad impairments on neuropsychological measures of executive function: a meta-analysis and review. Psychol Bull. 2013;139:81–132.
Rose EJ, Ebmeier KP. Pattern of impaired working memory during major depression. J Affect Disord. 2006;90:149–61.
Kurtz MM, Gerraty RT. A meta-analytic investigation of neurocognitive deficits in bipolar illness: profile and effects of clinical state. Neuropsychology. 2009;23:551–62.
Papmeyer M, Sussmann JE, Hall J, McKirdy J, Peel A, Macdonald A, et al. Neurocognition in individuals at high familial risk of mood disorders with or without subsequent onset of depression. Psychol Med. 2015;45:3317–27.
Adler CM, Holland SK, Schmithorst V, Tuchfarber MJ, Strakowski SM. Changes in neuronal activation in patients with bipolar disorder during performance of a working memory task. Bipolar Disord. 2004;6:540–9.
Thompson JM, Gray JM, Hughes JH, Watson S, Young AH, Ferrier IN. Impaired working memory monitoring in euthymic bipolar patients. Bipolar Disord. 2007;9:478–89.
Bauer IE, Jordan G, Soares JC, Meyer TD. The role of negative mood induction on working memory capacity in individuals putatively at risk for bipolar disorder: A pilot study. J Affect Disord. 2015;185:60–6.
Joormann J, Stanton CH. Examining emotion regulation in depression: A review and future directions. Behav Res Ther. 2016;86:35–49.
Townsend J, Altshuler LL. Emotion processing and regulation in bipolar disorder: a review. Bipolar Disord. 2012;14:326–39.
Phillips ML, Ladouceur CD, Drevets WC. A neural model of voluntary and automatic emotion regulation: implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Mol Psychiatry. 2008;13:829. 33-57
Bertocci MA, Bebko G, Dwojak A, Iyengar S, Ladouceur CD, Fournier JC, et al. Longitudinal relationships among activity in attention redirection neural circuitry and symptom severity in youth. Biol Psychiatry Cogn Neurosci Neuroimaging. 2017;2:336–45.
Fournier JC, Bertocci M, Ladouceur CD, Bonar L, Monk K, Abdul-Waalee H, et al. Neural function during emotion regulation and future depressive symptoms in youth at risk for affective disorders. Neuropsychopharmacology. 2021;46:1340–7.
Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci. 2011;15:483–506.
Raichle ME, Snyder AZ. A default mode of brain function: a brief history of an evolving idea. Neuroimage. 2007;37:1083–90.
Dai L, Zhou H, Xu X, Zuo Z. Brain structural and functional changes in patients with major depressive disorder: a literature review. PeerJ. 2019;7:e8170.
Wang X, Cheng B, Roberts N, Wang S, Luo Y, Tian F, et al. Shared and distinct brain fMRI response during performance of working memory tasks in adult patients with schizophrenia and major depressive disorder. Hum Brain Mapp. 2021;42:5458–76.
Park C, Rosenblat JD, Lee Y, Pan Z, Cao B, Iacobucci M, et al. The neural systems of emotion regulation and abnormalities in major depressive disorder. Behav Brain Res. 2019;367:181–8.
Yuksel D, Dietsche B, Konrad C, Dannlowski U, Kircher T, Krug A. Neural correlates of working memory in first episode and recurrent depression: An fMRI study. Prog Neuropsychopharmacol Biol Psychiatry. 2018;84:39–49.
Chase HW, Phillips ML. Elucidating neural network functional connectivity abnormalities in bipolar disorder: toward a harmonized methodological approach. Biol Psychiatry Cogn Neurosci Neuroimaging. 2016;1:288–98.
Bertocci MA, Afriyie-Agyemang Y, Rozovsky R, Iyengar S, Stiffler R, Aslam HA, et al. Altered patterns of central executive, default mode and salience network activity and connectivity are associated with current and future depression risk in two independent young adult samples. Mol Psychiatry. 2023;28:1046–56.
Green MJ, Cahill CM, Malhi GS. The cognitive and neurophysiological basis of emotion dysregulation in bipolar disorder. J Affect Disord. 2007;103:29–42.
Johnsen LK, Ver Loren van Themaat AH, Larsen KM, Burton BK, Baare WFC, Madsen KS, et al. Alterations in Task-Related Brain Activation in Children, Adolescents and Young Adults at Familial High-Risk for Schizophrenia or Bipolar Disorder - A Systematic Review. Front Psychiatry. 2020;11:632.
Chang K, Garrett A, Kelley R, Howe M, Sanders EM, Acquaye T, et al. Anomalous prefrontal-limbic activation and connectivity in youth at high-risk for bipolar disorder. J Affect Disord. 2017;222:7–13.
Han DH, Kim SM, Bae S, Renshaw PF, Anderson JS. A failure of suppression within the default mode network in depressed adolescents with compulsive internet game play. J Affect Disord. 2016;194:57–64.
Shapero BG, Chai XJ, Vangel M, Biederman J, Hoover CS, Whitfield-Gabrieli S, et al. Neural markers of depression risk predict the onset of depression. Psychiatry Res Neuroimaging. 2019;285:31–9.
Cassano GB, Dell’Osso L, Frank E, Miniati M, Fagiolini A, Shear K, et al. The bipolar spectrum: a clinical reality in search of diagnostic criteria and an assessment methodology. J Affect Disord. 1999;54:319–28.
Dell’Osso L, Armani A, Rucci P, Frank E, Fagiolini A, Corretti G, et al. Measuring mood spectrum: comparison of interview (SCI-MOODS) and self-report (MOODS-SR) instruments. Compr Psychiatry. 2002;43:69–73.
Cassano GB, Mula M, Rucci P, Miniati M, Frank E, Kupfer DJ, et al. The structure of lifetime manic-hypomanic spectrum. J Affect Disord. 2009;112:59–70.
Kelly RE Jr, Hoptman MJ. Replicability in Brain Imaging. Brain Sci. 2022;12:397.
Bertocci MA, Bebko GM, Mullin BC, Langenecker SA, Ladouceur CD, Almeida JR, et al. Abnormal anterior cingulate cortical activity during emotional n-back task performance distinguishes bipolar from unipolar depressed females. Psychol Med. 2012;42:1417–28.
Ladouceur CD, Silk JS, Dahl RE, Ostapenko L, Kronhaus DM, Phillips ML. Fearful faces influence attentional control processes in anxious youth and adults. Emotion. 2009;9:855–64.
Blair JR, Spreen O. Predicting premorbid IQ: A revision of the National Adult Reading Test. Clinical Neuropsychologist. 1989;3:129–36.
Annett M. A classification of hand preference by association analysis. Br J Psychol. 1970;61:303–21.
SAMHSA. National Survey on Drug Use and Health. Rockville, MD: Dept. of Health and Human Services, Substance Abuse and Mental Health Services Administration, Office of Applied Studies 2010.
First MB, Williams, JBW, Karg, RS, Spitzer, RL Structured Clinical Interview for DSM-5—Research Version (SCID-5 for DSM-5, Research Version; SCID-5-RV). American Psychiatric Association: Arlington, VA. 2015.
Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62.
Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32:50–5.
Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–35.
Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–9.
McLaren DG, Ries ML, Xu G, Johnson SC. A generalized form of context-dependent psychophysiological interactions (gPPI): a comparison to standard approaches. Neuroimage. 2012;61:1277–86.
Friedman J, Hastie, T, Simon, N, Tibshirani, R GLMNET. 2014.
Picard RR, Cook RD. Cross-validation of regression models. Journal of the American Statistical Association. 1984;79:575–83.
Bliss CI, Fisher RA. Fitting the negative binomial distribution to biological data. Biometrics. 1953;9:176–200.
NCSS. Negative binomial regression. NCSS Statistical Software; 2017.
Fransson P. Spontaneous low-frequency BOLD signal fluctuations: an fMRI investigation of the resting-state default mode of brain function hypothesis. Hum Brain Mapp. 2005;26:15–29.
Buckner RL. The brain’s default network: origins and implications for the study of psychosis. Dialogues Clin Neurosci. 2013;15:351–8.
Whitfield-Gabrieli S, Ford JM. Default mode network activity and connectivity in psychopathology. Annu Rev Clin Psychol. 2012;8:49–76.
Luca M. Maladaptive Rumination as a Transdiagnostic Mediator of Vulnerability and Outcome in Psychopathology. J Clin Med. 2019;8:314.
Bartova L, Meyer BM, Diers K, Rabl U, Scharinger C, Popovic A, et al. Reduced default mode network suppression during a working memory task in remitted major depression. J Psychiatr Res. 2015;64:9–18.
Zhou HX, Chen X, Shen YQ, Li L, Chen NX, Zhu ZC, et al. Rumination and the default mode network: Meta-analysis of brain imaging studies and implications for depression. Neuroimage. 2020;206:116287.
Rodriguez-Cano E, Alonso-Lana S, Sarro S, Fernandez-Corcuera P, Goikolea JM, Vieta E, et al. Differential failure to deactivate the default mode network in unipolar and bipolar depression. Bipolar Disord. 2017;19:386–95.
Fernandez-Corcuera P, Salvador R, Monte GC, Salvador Sarro S, Goikolea JM, Amann B, et al. Bipolar depressed patients show both failure to activate and failure to de-activate during performance of a working memory task. J Affect Disord. 2013;148:170–8.
LeMoult J, Gotlib IH. Depression: A cognitive perspective. Clin Psychol Rev. 2019;69:51–66.
Disner SG, Shumake JD, Beevers CG. Self-referential schemas and attentional bias predict severity and naturalistic course of depression symptoms. Cogn Emot. 2017;31:632–44.
Nolen-Hoeksema S, Wisco BE, Lyubomirsky S. Rethinking Rumination. Perspect Psychol Sci. 2008;3:400–24.
Nolen-Hoeksema S, Morrow J, Fredrickson BL. Response styles and the duration of episodes of depressed mood. Journal of Abnormal Psychology. 1993;102:569–82.
Just N, Alloy LB. The response styles theory of depression: tests and an extension of the theory. J Abnorm Psychol. 1997;106:221–9.
Curci A, Lanciano T, Soleti E, Rime B. Negative emotional experiences arouse rumination and affect working memory capacity. Emotion. 2013;13:867–80.
Stout DM, Bomyea J, Risbrough VB, Simmons AN. Aversive distractors modulate affective working memory in frontoparietal regions. Emotion. 2020;20:286–95.
Levens SM, Muhtadie L, Gotlib IH. Rumination and impaired resource allocation in depression. J Abnorm Psychol. 2009;118:757–66.
Bruning AL, Mallya MM, Lewis-Peacock JA. Rumination burdens the updating of working memory. Atten Percept Psychophys. 2023;85:1452–60.
McGrogan CL, Dodd AL, Smith MA. Emotion regulation strategies in mania risk: A systematic review. J Clin Psychol. 2019;75:2106–18.
Gruber J, Eidelman P, Johnson SL, Smith B, Harvey AG. Hooked on a feeling: rumination about positive and negative emotion in inter-episode bipolar disorder. J Abnorm Psychol. 2011;120:956–61.
Feldman GC, Joormann J, Johnson SL. Responses to Positive Affect: A Self-Report Measure of Rumination and Dampening. Cognit Ther Res. 2008;32:507–25.
Watkins ER. Constructive and unconstructive repetitive thought. Psychol Bull. 2008;134:163–206.
Ghaznavi S, Chou T, Dougherty DD, Nierenberg AA. Differential patterns of default mode network activity associated with negative and positive rumination in bipolar disorder. J Affect Disord. 2023;323:607–16.
Nolen-Hoeksema S. The role of rumination in depressive disorders and mixed anxiety/depressive symptoms. J Abnorm Psychol. 2000;109:504–11.
Watkins ER, Roberts H. Reflecting on rumination: Consequences, causes, mechanisms and treatment of rumination. Behav Res Ther. 2020;127:103573.
Spasojević J, Alloy LB. Rumination as a common mechanism relating depressive risk factors to depression. Emotion. 2001;1:25–37.
Vieta E, Berk M, Schulze TG, Carvalho AF, Suppes T, Calabrese JR, et al. Bipolar disorders. Nat Rev Dis Primers. 2018;4:18008.
Kerestes R, Ladouceur CD, Meda S, Nathan PJ, Blumberg HP, Maloney K, et al. Abnormal prefrontal activity subserving attentional control of emotion in remitted depressed patients during a working memory task with emotional distracters. Psychol Med. 2012;42:29–40.
Walter H, Wolf RC, Spitzer M, Vasic N. Increased left prefrontal activation in patients with unipolar depression: an event-related, parametric, performance-controlled fMRI study. J Affect Disord. 2007;101:175–85.
Phillips ML, Swartz HA. A critical appraisal of neuroimaging studies of bipolar disorder: toward a new conceptualization of underlying neural circuitry and a road map for future research. Am J Psychiatry. 2014;171:829–43.
Dolcos F, McCarthy G. Brain systems mediating cognitive interference by emotional distraction. J Neurosci. 2006;26:2072–9.
Migo M, Simpson K, Peters A, Ellard KK, Chou T, Nierenberg AA, et al. Dimensional Affective Processing in BD. Psychiatry Res. 2022;307:114304.
Heissler J, Kanske P, Schonfelder S, Wessa M. Inefficiency of emotion regulation as vulnerability marker for bipolar disorder: evidence from healthy individuals with hypomanic personality. J Affect Disord. 2014;152-154:83–90.
Adamczyk AK, Wyczesany M, van Peer JM. High working memory load impairs reappraisal but facilitates distraction - An event-related potential investigation. Biol Psychol. 2022;171:108327.
Funding
This study was supported by R01MH100041 and R37MH100041 (PI: M.L.P.) from the National Institute of Mental Health, the Pittsburgh Foundation (PI: M.L.P.), and the Brain and Behavior Research Foundation (PI: M.A.B.). This research was supported in part by the University of Pittsburgh Center for Research Computing, RRID:SCR_022735, through the resources provided. Specifically, this work used the HTC cluster, which is supported by NIH award number S10OD028483. The funding agency was not involved in the conduct, analysis, or reporting of this work.
Author information
Authors and Affiliations
Contributions
YA-A, MAB and MLP conceived of and wrote the manuscript. YA-A completed the analyses. YA-A, MAB, SI, and MLP conceived of and interpreted the statistical analysis. RS, LB, HAA, SG, GB, OB and YW contributed substantially to the acquisition of the data. AS, TY and HC contributed substantially to the processing of the data. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
Afriyie-Agyemang, Bertocci, Iyengar, Stiffler, Bonar, Aslam, Graur, Bebko, Skeba, Brady, Benjamin, Wang Chase, Phillips have no financial interests or potential conflicts of interest.
Ethics approval and consent to participate
All methods were performed in accordance with the Declaration of Helsinki. The University of Pittsburgh Institutional Review Board approved this study (IRB no: 18110024, 19040176). All participants gave written informed consent.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Afriyie-Agyemang, Y., Bertocci, M.A., Iyengar, S. et al. Lifetime depression and mania/hypomania risk predicted by neural markers in three independent young adult samples during working memory and emotional regulation. Mol Psychiatry 30, 870–880 (2025). https://doi.org/10.1038/s41380-024-02702-6
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41380-024-02702-6