Abstract
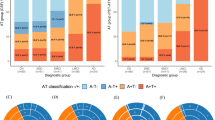
Cognitive dysfunction in Alzheimer’s disease (AD) correlates closely with pathology in the neuronal microtubule-associated protein tau. Tau pathology may spread via neural synapses. In a population of cognitively unimpaired elderly at elevated risk of AD, we investigated four cerebrospinal (CSF) markers of synaptic dysfunction and degeneration. Three of these (SYT1, SNAP25, and ADAM23) are derived from pre-synaptic structures, while ADAM22 reflects post-synaptic changes. All four markers correlated strongly with tau protein measures. In statistical models, SYT1 accounted for more than half the total variance in both total- and P(181)-tau levels. Observed correlations with CSF levels of Alzheimer amyloid-β (Aβ42) were somewhat weaker. In longitudinal data, baseline levels of ADAM22 and ADAM23 robustly predicted increase over time in both total- and P-tau. CSF SYT1 levels also correlated with PET image uptake of tau and (at a trend level) Aβ in areas of interest for early AD pathology. CSF SYT1 and SNAP25 levels correlated inversely with a global psychometric score and several of its domain subscales. In quantitative trait loci analyses, all four synaptic markers were associated with at least one AD genetic risk locus. Upon “staging” participants by their evidence of amyloid and tau pathology (A/T/N framework), the CSF synaptic markers were unexpectedly reduced in participants with CSF evidence of amyloid but not tau pathology. They were clearly elevated, however, in the CSF of persons with indications of both tau and amyloid pathology. These observations provide evidence for clear pre-synaptic degeneration in cognitively unimpaired persons with biomarker evidence of early AD pathology.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Figures data has been archived at https://doi.org/10.5281/zenodo.14003860. The multimodal data, including subject characteristics, CSF biomarkers levels, tau and amyloid-PET SUVR, APOE genotypes, and RBANS scores, have been summarized in the supporting data files from the PREVENT-AD data release 7. Other datasets are available from the corresponding authors upon reasonable request.
References
Wimo A, Seeher K, Cataldi R, Cyhlarova E, Dielemann JL, Frisell O, et al. The worldwide costs of dementia in 2019. Alzheimer’s Dement. 2023;19:2865–73.
Bali J, Gheinani AH, Zurbriggen S, Rajendran L. Role of genes linked to sporadic Alzheimer’s disease risk in the production of β-amyloid peptides. Proc Natl Acad Sci USA. 2012;109:15307–11.
Cummings J, Aisen P, Lemere C, Atri A, Sabbagh M, Salloway S. Aducanumab produced a clinically meaningful benefit in association with amyloid lowering. Alzheimer’s Res Ther. 2021;13:98.
Swanson CJ, Zhang Y, Dhadda S, Wang J, Kaplow J, Lai RYK, et al. A randomized, double-blind, phase 2b proof-of-concept clinical trial in early Alzheimer’s disease with lecanemab, an anti-Aβ protofibril antibody. Alzheimer’s Res Ther. 2021;13:80.
Tampi RR, Forester BP, Agronin M. Aducanumab: evidence from clinical trial data and controversies. Drugs Context 2021;10:2021–2027.
Colom-Cadena M, Spires-Jones T, Zetterberg H, Blennow K, Caggiano A, DeKosky ST, et al. The clinical promise of biomarkers of synapse damage or loss in Alzheimer’s disease. Alzheimer’s Res Ther. 2020;12:21.
DeKosky ST, Scheff SW. Synapse loss in frontal cortex biopsies in Alzheimer’s disease: correlation with cognitive severity. Ann Neurol. 1990;27:457–64.
Mecca AP, Chen M-K, O’Dell RS, Naganawa M, Toyonaga T, Godek TA, et al. In vivo measurement of widespread synaptic loss in Alzheimer’s disease with SV2A PET. Alzheimer’s Dement. 2020;16:974–82.
Brier MR, Gordon B, Friedrichsen K, McCarthy J, Stern A, Christensen J, et al. Tau and Aβ imaging, CSF measures, and cognition in Alzheimer’s disease. Sci Transl Med. 2016;8:338ra366.
Sperling RA, Mormino EC, Schultz AP, Betensky RA, Papp KV, Amariglio RE, et al. The impact of amyloid-beta and tau on prospective cognitive decline in older individuals. Ann Neurol. 2019;85:181–93.
Taddei RN, Perbet R, Mate de Gerando A, Wiedmer AE, Sanchez-Mico M, Connors Stewart T, et al. Tau oligomer–containing synapse elimination by microglia and astrocytes in Alzheimer disease. JAMA Neurol. 2023;80:1209–21.
Arnsten AFT, Datta D, Del Tredici K, Braak H. Hypothesis: tau pathology is an initiating factor in sporadic Alzheimer’s disease. Alzheimers Dement. 2021;17:115–24.
Franzmeier N, Dehsarvi A, Steward A, Biel D, Dewenter A, Roemer SN, et al. Elevated CSF GAP-43 is associated with accelerated tau accumulation and spread in Alzheimer’s disease. Nat Commun. 2024;15:202.
Hong S, Beja-Glasser VF, Nfonoyim BM, Frouin A, Li S, Ramakrishnan S, et al. Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science. 2016;352:712–6.
Bie B, Wu J, Foss JF, Naguib M. An overview of the cannabinoid type 2 receptor system and its therapeutic potential. Curr Opin Anaesthesiol. 2018;31:407–14.
Dejanovic B, Huntley MA, De Mazière A, Meilandt WJ, Wu T, Srinivasan K, et al. Changes in the synaptic proteome in tauopathy and rescue of tau-induced synapse loss by C1q antibodies. Neuron. 2018;100:1322–36.e1327.
Shi Q, Chowdhury S, Ma R, Le KX, Hong S, Caldarone BJ, et al. Complement C3 deficiency protects against neurodegeneration in aged plaque-rich APP/PS1 mice. Sci Transl Med. 2017;9:eaaf6295.
Edwards DR, Handsley MM, Pennington CJ. The ADAM metalloproteinases. Mol Asp Med. 2008;29:258–89.
Hsia H-E, Tüshaus J, Brummer T, Zheng Y, Scilabra SD, Lichtenthaler SF. Functions of ‘A disintegrin and metalloproteases (ADAMs)’ in the mammalian nervous system. Cell Mol Life Sci. 2019;76:3055–81.
Whelan CD, Mattsson N, Nagle MW, Vijayaraghavan S, Hyde C, Janelidze S, et al. Multiplex proteomics identifies novel CSF and plasma biomarkers of early Alzheimer’s disease. Acta Neuropathol Commun. 2019;7:169.
Llaurador-Coll M, Rios S, García-Gavilán JF, Babio N, Vilella E, Salas-Salvadó J. Plasma levels of neurology-related proteins are associated with cognitive performance in an older population with overweight/obesity and metabolic syndrome. Geroscience. 2023;45:2457–70.
Brinkmalm A, Brinkmalm G, Honer WG, Frölich L, Hausner L, Minthon L, et al. SNAP-25 is a promising novel cerebrospinal fluid biomarker for synapse degeneration in Alzheimer’s disease. Mol Neurodegener. 2014;9:53.
Brinkmalm A, Dumurgier J, Brinkmalm G, Hansson O, Zetterberg H, Bouaziz-Amar E, et al. The pre-synaptic vesicle protein synaptotagmin is a novel biomarker for Alzheimer’s disease. Alzheimer’s Res Ther. 2016;8:41.
Wolfes AC, Dean C. The diversity of synaptotagmin isoforms. Curr Opin Neurobiol. 2020;63:198–209.
Antonucci F, Corradini I, Fossati G, Tomasoni R, Menna E, Matteoli M. SNAP-25, a known presynaptic protein with emerging postsynaptic functions. Front Synaptic Neurosci. 2016;8:7.
Breitner J, Poirier J, Etienne P, Leoutsakos J. Rationale and structure for a new center for studies on prevention of Alzheimer’s disease (StoP-AD). J Prev Alzheimer’s Dis. 2016;3:236–42.
Picard C, Nilsson N, Labonté A, Auld D, Rosa-Neto P, Initiative tAsDN, et al. Apolipoprotein B is a novel marker for early tau pathology in Alzheimer’s disease. Alzheimer’s Dement. 2022;18:875–87.
Lleó A, Alcolea D, Martínez-Lage P, Scheltens P, Parnetti L, Poirier J, et al. Longitudinal cerebrospinal fluid biomarker trajectories along the Alzheimer’s disease continuum in the BIOMARKAPD study. Alzheimer’s Dement. 2019;15:742–53.
McSweeney M, Pichet Binette A, Meyer PF, Gonneaud J, Bedetti C, Ozlen H, et al. Intermediate flortaucipir uptake is associated with Aβ-PET and CSF tau in asymptomatic adults. Neurology. 2020;94:e1190–200.
Ossenkoppele R, Rabinovici GD, Smith R, Cho H, Schöll M, Strandberg O, et al. Discriminative accuracy of [18F] flortaucipir positron emission tomography for Alzheimer disease vs other neurodegenerative disorders. JAMA. 2018;320:1151–62.
Randolph C, Tierney MC, Mohr E, Chase TN. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): preliminary clinical validity. J Clin Exp Neuropsychol. 1998;20:310–9.
Jack CR Jr., Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, et al. NIA-AA research framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018;14:535–62.
Tijms BM, Bertens D, Slot RE, Gouw AA, Teunissen CE, Scheltens P, et al. Low normal cerebrospinal fluid Aβ42 levels predict clinical progression in nondemented subjects. Ann Neurol. 2017;81:749–53.
Farrell ME, Kennedy KM, Rodrigue KM, Wig G, Bischof GN, Rieck JR, et al. Association of longitudinal cognitive decline with amyloid burden in middle-aged and older adults: evidence for a dose-response relationship. JAMA Neurol. 2017;74:830–8.
Donohue MC, Sperling RA, Petersen R, Sun C-K, Weiner MW, Aisen PS, et al. Association between elevated brain amyloid and subsequent cognitive decline among cognitively normal persons. JAMA. 2017;317:2305–16.
Meyer PF, Savard M, Poirier J, Labonté A, Rosa-Neto P, Weitz TM, et al. Bi-directional association of cerebrospinal fluid immune markers with stage of Alzheimer’s disease pathogenesis. J Alzheimers Dis. 2018;63:577–90.
Hu X, Pickering E, Liu YC, Hall S, Fournier H, Katz E, et al. Meta-analysis for genome-wide association study identifies multiple variants at the BIN1 locus associated with late-onset Alzheimer’s disease. PLoS ONE. 2011;6:e16616.
Tremblay M, Vezina H. Genealogical analysis of maternal and paternal lineages in the Quebec population. Hum Biol. 2010;82:179–98.
Peltonen L, Palotie A, Lange K. Use of population isolates for mapping complex traits. Nat Rev Genet. 2000;1:182–90.
Goossens J, Cervantes González A, Dewit N, Lidón L, Fortea J, Alcolea D, et al. Evaluation of cerebrospinal fluid levels of synaptic vesicle protein, VAMP-2, across the sporadic Alzheimer’s disease continuum. Alzheimer’s Res Ther. 2023;15:186.
Sandelius Å, Portelius E, Källén Å, Zetterberg H, Rot U, Olsson B, et al. Elevated CSF GAP-43 is Alzheimer’s disease specific and associated with tau and amyloid pathology. Alzheimer’s Dement. 2019;15:55–64.
Tzioras M, Daniels MJD, Davies C, Baxter P, King D, McKay S, et al. Human astrocytes and microglia show augmented ingestion of synapses in Alzheimer’s disease via MFG-E8. Cell Rep Med. 2023;4:101175.
Bellenguez C, Küçükali F, Jansen IE, Kleineidam L, Moreno-Grau S, Amin N, et al. New insights into the genetic etiology of Alzheimer’s disease and related dementias. Nat Genet. 2022;54:412–36.
Lüscher C, Malenka RC. NMDA receptor-dependent long-term potentiation and long-term depression (LTP/LTD). Cold Spring Harb Perspect Biol. 2012;4:a005710.
Alkadhi KA. NMDA receptor-independent LTP in mammalian nervous system. Prog Neurobiol. 2021;200:101986.
Chen QS, Kagan BL, Hirakura Y, Xie CW. Impairment of hippocampal long‐term potentiation by Alzheimer amyloid β‐peptides. J Neurosci Res. 2000;60:65–72.
Walsh DM, Klyubin I, Fadeeva JV, Cullen WK, Anwyl R, Wolfe MS, et al. Naturally secreted oligomers of amyloid β protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002;416:535–9.
Zetterberg H, Bendlin BB. Biomarkers for Alzheimer’s disease-preparing for a new era of disease-modifying therapies. Mol Psychiatry. 2021;26:296–308.
Wu JW, Hussaini SA, Bastille IM, Rodriguez GA, Mrejeru A, Rilett K, et al. Neuronal activity enhances tau propagation and tau pathology in vivo. Nat Neurosci. 2016;19:1085–92.
Busche MA, Eichhoff G, Adelsberger H, Abramowski D, Wiederhold KH, Haass C, et al. Clusters of hyperactive neurons near amyloid plaques in a mouse model of Alzheimer’s disease. Science. 2008;321:1686–9.
Kehoe PG, Al Mulhim N, Zetterberg H, Blennow K, Miners JS. Cerebrospinal fluid changes in the renin-angiotensin system in Alzheimer’s disease. J Alzheimers Dis. 2019;72:525–35.
Jochemsen HM, Teunissen CE, Ashby EL, van der Flier WM, Jones RE, Geerlings MI, et al. The association of angiotensin-converting enzyme with biomarkers for Alzheimer’s disease. Alzheimer’s Res Ther. 2014;6:27.
Acknowledgements
The authors would like to thank Mrs. Jennifer Tremblay-Mercier, Doris Dea, and Louise Théroux for their individual contributions at different stages of the project. The authors would also like to thank Drs. Lisa Munter and Patricia Silveira for their technical expertise. The funding agency played no role in the conduct of the study. JP is supported by the Fonds de recherche en santé du Québec (FRSQ), the CIHR (# PJT 153287, 178210), the Natural Sciences and Engineering Research Council of Canada (NSERC), and the J.L. Levesque Foundation. SV is supported by the FRQS, the CIHR (PJT: 178385, 438655), and Brain Canada whereas JCB is supported by CIHR. HZ is a Wallenberg Scholar and a Distinguished Professor at the Swedish Research Council supported by grants from the Swedish Research Council (#2023-00356; #2022-01018 and #2019-02397), the European Union’s Horizon Europe research and innovation programme under grant agreement No 101053962, Swedish State Support for Clinical Research (#ALFGBG-71320), the Alzheimer Drug Discovery Foundation (ADDF), USA (#201809-2016862), the AD Strategic Fund and the Alzheimer’s Association (#ADSF-21-831376-C, #ADSF-21-831381-C, #ADSF-21-831377-C, and #ADSF-24-1284328-C), the Bluefield Project, Cure Alzheimer’s Fund, the Olav Thon Foundation, the Erling-Persson Family Foundation, Familjen Rönströms Stiftelse, Stiftelsen för Gamla Tjänarinnor, Hjärnfonden, Sweden (#FO2022-0270), the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No 860197 (MIRIADE), the European Union Joint Programme – Neurodegenerative Disease Research (JPND2021-00694), the National Institute for Health and Care Research University College London Hospitals Biomedical Research Centre, and the UK Dementia Research Institute at UCL (UKDRI-1003). KB is supported by the Swedish Research Council (#2017-00915 and #2022-00732), the Swedish Alzheimer Foundation (#AF-930351, #AF-939721, #AF-968270, and #AF-994551), Hjärnfonden, Sweden (#FO2017-0243 and #ALZ2022-0006), the Swedish state under the agreement between the Swedish government and the County Councils, the ALF-agreement (#ALFGBG-715986 and #ALFGBG-965240), the European Union Joint Program for Neurodegenerative Disorders (JPND2019-466-236), the Alzheimer’s Association 2021 Zenith Award (ZEN-21-848495), the Alzheimer’s Association 2022-2025 Grant (SG-23-1038904 QC), La Fondation Recherche Alzheimer (FRA), Paris, France, the Kirsten and Freddy Johansen Foundation, Copenhagen, Denmark, and Familjen Rönströms Stiftelse, Stockholm, Sweden. Data used in preparation of this article were obtained from the program of PRe-symptomatic EValuation of Novel or Experimental Treatments for Alzheimer’s Disease (PREVENT-AD) at the Centre for Studies on Prevention of Alzheimer’s Disease (StoP-AD), Douglas Mental Health University Institute Research Center (http://douglas.research.mcgill.ca/stop-ad-centre). A complete listing of the PREVENT-AD Research Group can be found at: https://preventad.loris.ca/acknowledgements/acknowledgements.php?date=2024-12-30.
Author information
Authors and Affiliations
Consortia
Contributions
JA, JP, CP, SV, JB, KB, and HZ conceptualized the research. CP, JA, AB, HZ, and KB performed CSF biomarkers measurements, data quality control, and data compilation. DA, CP, and JA performed the pan-genomic analysis of DNA samples and risk assessments. JP, JA, CP, AB, SV, and JB contributed to the data analysis. JP, JA, CP, SV, and CP developed the algorithms for data analysis. JA, JP, JB, and SV wrote the original manuscript draft. All authors reviewed, edited, and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
JP serves as a scientific advisor to the Alzheimer Society of France. HZ has served at scientific advisory boards and/or as a consultant for Abbvie, Acumen, Alector, Alzinova, ALZPath, Amylyx, Annexon, Apellis, Artery Therapeutics, AZTherapies, Cognito Therapeutics, CogRx, Denali, Eisai, Merry Life, Nervgen, Novo Nordisk, Optoceutics, Passage Bio, Pinteon Therapeutics, Prothena, Red Abbey Labs, reMYND, Roche, Samumed, Siemens Healthineers, Triplet Therapeutics, and Wave, has given lectures in symposia sponsored by Alzecure, Biogen, Cellectricon, Fujirebio, Lilly, Novo Nordisk, and Roche, and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program (outside submitted work).KB has served as a consultant and on advisory boards for Abbvie, AC Immune, ALZPath, AriBio, BioArctic, Biogen, Eisai, Lilly, and Moleac Pte. Ltd, Neurimmune, Novartis, Ono Pharma, Prothena, Roche Diagnostics, and Siemens Healthineers; has served on data monitoring committees for Julius Clinical and Novartis; has given lectures, produced educational materials, and participated in educational programs for AC Immune, Biogen, Celdara Medical, Eisai and Roche Diagnostics; and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program, outside the work presented in this paper. All other authors have nothing to disclose.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ao, J., Picard, C., Auld, D. et al. Novel synaptic markers predict early tau pathology and cognitive deficit in an asymptomatic population at risk of Alzheimer’s disease. Mol Psychiatry 30, 2810–2820 (2025). https://doi.org/10.1038/s41380-024-02884-z
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41380-024-02884-z