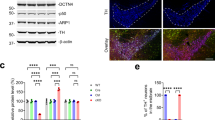
Abstract
Age-related dopamine (DA) neuron loss is a primary feature of Parkinson’s disease. However, whether similar biological processes occur during healthy aging, but to a lesser degree, remains unclear. We therefore determined whether midbrain DA neurons degenerate during aging in mice and humans. In mice, we identified no difference in midbrain neuron numbers throughout aging. Despite this, we found age-related decreases in midbrain mRNA expression of tyrosine hydroxylase (Th), the rate limiting enzyme of DA synthesis. Among midbrain glutamatergic cells, we similarly identified age-related declines in vesicular glutamate transporter 2 (Vglut2) mRNA expression. In co-transmitting Th+/Vglut2+ neurons, Th and Vglut2 transcripts decreased with aging. However, Th and Vglut2 protein levels in striatal synaptic release sites (e.g., terminals and axonal projections) did not differ throughout aging. Similar to the mouse, an initial study of human brain showed no effect of aging on midbrain neuron number with a concomitant decrease in TH and VGLUT2 mRNA expression. Unlike in mice, the density of striatal TH+ dopaminergic terminals was lower in aged human subjects. However, TH and VGLUT2 protein levels were unaffected in the remaining striatal boutons. Finally, in contrast to Th and Vglut2 mRNA, expression of most ribosomal genes in Th+ neurons was either maintained or even upregulated during aging. This suggests a homeostatic mechanism where age-related declines in transcriptional efficiency are overcome by ongoing ribosomal translation. Overall, we demonstrate species-conserved transcriptional effects of aging in midbrain dopaminergic and glutamatergic neurons that are not accompanied by marked cell death or lower striatal protein expression. This opens the door to novel therapeutic approaches to maintain neurotransmission and bolster neuronal resilience.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Transcriptomic analyses employed previously deposited RNA sequencing data (GSE129788). Customized computer code has been deposited in Github and can be accessed at: https://github.com/tsbanks16/Aging_Paper.
References
Sulzer D, Surmeier DJ. Neuronal vulnerability, pathogenesis, and Parkinson’s disease. Mov Disord. 2013;28:715–24.
Reeve A, Simcox E, Turnbull D. Ageing and Parkinson’s disease: why is advancing age the biggest risk factor? Ageing Res Rev. 2014;14:19–30.
Lee J, Kim HJ. Normal aging induces changes in the brain and neurodegeneration progress: review of the structural, biochemical, metabolic, cellular, and molecular changes. Front Aging Neurosci. 2022;14:931536.
Ota M, Yasuno F, Ito H, Seki C, Nozaki S, Asada T, et al. Age-related decline of dopamine synthesis in the living human brain measured by positron emission tomography with L-[beta-11C]DOPA. Life Sci. 2006;79:730–6.
Volkow ND, Ding YS, Fowler JS, Wang GJ, Logan J, Gatley SJ, et al. Dopamine transporters decrease with age. J Nucl Med. 1996;37:554–9.
Kaasinen V, Vilkman H, Hietala J, Någren K, Helenius H, Olsson H, et al. Age-related dopamine D2/D3 receptor loss in extrastriatal regions of the human brain. Neurobiol Aging. 2000;21:683–8.
Coleman CR, Pallos J, Arreola-Bustos A, Wang L, Raftery D, Promislow DEL, et al. Natural variation in age-related dopamine neuron degeneration is glutathione-dependent and linked to life span. bioRxiv. 2024; https://doi.org/10.1101/2024.02.12.580013.
Noda S, Sato S, Fukuda T, Tada N, Hattori N. Aging-related motor function and dopaminergic neuronal loss in C57BL/6 mice. Mol Brain. 2020;13:46.
Lebowitz JJ, Khoshbouei H. Heterogeneity of dopamine release sites in health and degeneration. Neurobiol Dis. 2020;134:104633.
Dickerson JW, Hemmerle AM, Numan S, Lundgren KH, Seroogy KB. Decreased expression of ErbB4 and tyrosine hydroxylase mRNA and protein in the ventral midbrain of aged rats. Neuroscience. 2009;163:482–9.
Emborg ME, Ma SY, Mufson EJ, Levey AI, Taylor MD, Brown WD, et al. Age-related declines in nigral neuronal function correlate with motor impairments in rhesus monkeys. J Comp Neurol. 1998;401:253–65.
Siddiqi Z, Kemper TL, Killiany R. Age-related neuronal loss from the substantia nigra-pars compacta and ventral tegmental area of the rhesus monkey. J Neuropathol Exp Neurol. 1999;58:959–71.
Bannon MJ, Poosch MS, Xia Y, Goebel DJ, Cassin B, Kapatos G. Dopamine transporter mRNA content in human substantia nigra decreases precipitously with age. Proc Natl Acad Sci. 1992;89:7095–9.
Bannon MJ, Whitty CJ. Age-related and regional differences in dopamine transporter mRNA expression in human midbrain. Neurology. 1997;48:969–77.
Fearnley JM, Lees AJ. Ageing and Parkinson’s disease: substantia nigra regional selectivity. Brain. 1991;114:2283–301.
Gibb WR, Lees AJ. Anatomy, pigmentation, ventral and dorsal subpopulations of the substantia nigra, and differential cell death in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1991;54:388–96.
Ma SY, Roytt M, Collan Y, Rinne JO. Unbiased morphometrical measurements show loss of pigmented nigral neurones with ageing. Neuropathol Appl Neurobiol. 1999;25:394–9.
Muthane U, Yasha TC, Shankar SK. Low numbers and no loss of melanized nigral neurons with increasing age in normal human brains from India. Ann Neurol. 1998;43:283–7.
Kubis N, Faucheux BA, Ransmayr G, Damier P, Duyckaerts C, Henin D, et al. Preservation of midbrain catecholaminergic neurons in very old human subjects. Brain. 2000;123:366–73.
Cabello CR, Thune JJ, Pakkenberg H, Pakkenberg B. Ageing of substantia nigra in humans: cell loss may be compensated by hypertrophy. Neuropathol Appl Neurobiol. 2002;28:283–91.
Chu Y, Kompoliti K, Cochran EJ, Mufson EJ, Kordower JH. Age-related decreases in Nurr1 immunoreactivity in the human substantia nigra. J Comp Neurol. 2002;450:203–14.
Pakkenberg B, Møller A, Gundersen HJ, Mouritzen Dam A, Pakkenberg H. The absolute number of nerve cells in substantia nigra in normal subjects and in patients with Parkinson’s disease estimated with an unbiased stereological method. J Neurol Neurosurg Psychiatry. 1991;54:30–33.
Lin KJ, Weng YH, Hsieh CJ, Lin WY, Wey SP, Kung MP, et al. Brain imaging of vesicular monoamine transporter type 2 in healthy aging subjects by 18F-FP-(+)-DTBZ PET. PLoS ONE. 2013;8:e75952.
Hall FS, Itokawa K, Schmitt A, Moessner R, Sora I, Lesch KP, et al. Decreased vesicular monoamine transporter 2 (VMAT2) and dopamine transporter (DAT) function in knockout mice affects aging of dopaminergic systems. Neuropharmacology. 2014;76:146–55.
Sun Y, Li YS, Li B, Ma K, Li BX. A study of the age-related effects of lactational atrazine exposure. Reprod Toxicol. 2017;69:230–41.
Buccitelli C, Selbach M. mRNAs, proteins and the emerging principles of gene expression control. Nat Rev Genet. 2020;21:630–44.
Vogel C, Marcotte EM. Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat Rev Genet. 2012;13:227–32.
Buck SA, Steinkellner T, Aslanoglou D, Villeneuve M, Bhatte SH, Childers VC, et al. Vesicular glutamate transporter modulates sex differences in dopamine neuron vulnerability to age-related neurodegeneration. Aging Cell. 2021;20:e13365.
Flurkey K, Currer J, Harrison D. Mouse models in aging research. The Mouse in Biomedical Res. 2007;3:637–72.
Buck SA, Miranda BR, Logan RW, Fish KN, Greenamyre JT, Freyberg Z. VGLUT2 is a determinant of dopamine neuron resilience in a rotenone model of dopamine neurodegeneration. J Neurosci. 2021;41:4937–47.
Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. Animal research: reporting in vivo experiments: the ARRIVE guidelines. J Gene Med. 2010;12:561–3.
Drummond GB, Paterson DJ, McGrath JC. ARRIVE: new guidelines for reporting animal research. Exp Physiol. 2010;95:841.
Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol. 2010;8:e1000412.
Kelly TM, Mann JJ. Validity of DSM-III-R diagnosis by psychological autopsy: a comparison with clinician ante-mortem diagnosis. Acta Psychiatr Scand. 1996;94:337–43.
Lewis DA. The human brain revisited: opportunities and challenges in postmortem studies of psychiatric disorders. Neuropsychopharmacology. 2002;26:143–54.
Deep-Soboslay A, Akil M, Martin CE, Bigelow LB, Herman MM, Hyde TM, et al. Reliability of psychiatric diagnosis in postmortem research. Biol Psychiatry. 2005;57:96–101.
Glausier JR, Kelly MA, Salem S, Chen K, Lewis DA. Proxy measures of premortem cognitive aptitude in postmortem subjects with schizophrenia. Psychol Med. 2020;50:507–14.
Glantz LA, Lewis DA. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch Gen Psychiatry. 2000;57:65–73.
Motulsky HJ, Brown RE. Detecting outliers when fitting data with nonlinear regression - a new method based on robust nonlinear regression and the false discovery rate. BMC Bioinforma. 2006;7:123.
Paxinos G, Franklin KBJ The Mouse Brain in Stereotaxic Coordinates. San Diego: Academic Press; 2001.
Curley AA, Arion D, Volk DW, Asafu-Adjei JK, Sampson AR, Fish KN, et al. Cortical deficits of glutamic acid decarboxylase 67 expression in schizophrenia: clinical, protein, and cell type-specific features. Am J Psychiatry. 2011;168:921–9.
Glausier JR, Fish KN, Lewis DA. Altered parvalbumin basket cell inputs in the dorsolateral prefrontal cortex of schizophrenia subjects. Mol Psychiatry. 2014;19:30–36.
Rocco BR, Lewis DA, Fish KN. Markedly lower glutamic acid decarboxylase 67 protein levels in a subset of boutons in schizophrenia. Biol Psychiatry. 2016;79:1006–15.
Rocco BR, Sweet RA, Lewis DA, Fish KN. GABA-synthesizing enzymes in calbindin and calretinin neurons in monkey prefrontal cortex. Cereb Cortex. 2016;26:2191–204.
Zhang S, Qi J, Li X, Wang HL, Britt JP, Hoffman AF, et al. Dopaminergic and glutamatergic microdomains in a subset of rodent mesoaccumbens axons. Nat Neurosci. 2015;18:386–92.
Herzog E, Takamori S, Jahn R, Brose N, Wojcik SM. Synaptic and vesicular co-localization of the glutamate transporters VGLUT1 and VGLUT2 in the mouse hippocampus. J Neurochem. 2006;99:1011–8.
Chen SY, Lu KM, Ko HA, Huang TH, Hao JH, Yan YT, et al. Parcellation of the striatal complex into dorsal and ventral districts. Proc Natl Acad Sci. 2020;117:7418–29.
Fish KN, Sweet RA, Deo AJ, Lewis DA. An automated segmentation methodology for quantifying immunoreactive puncta number and fluorescence intensity in tissue sections. Brain Res. 2008;1240:62–72.
Ximerakis M, Lipnick SL, Innes BT, Simmons SK, Adiconis X, Dionne D, et al. Single-cell transcriptomic profiling of the aging mouse brain. Nat Neurosci. 2019;22:1696–708.
Wickham H. ggplot2: Elegant Graphics for Data Analysis. New York: Springer International Publishing; 2009.
Poulin JF, Caronia G, Hofer C, Cui Q, Helm B, Ramakrishnan C, et al. Mapping projections of molecularly defined dopamine neuron subtypes using intersectional genetic approaches. Nat Neurosci. 2018;21:1260–71.
Trudeau LE, Hnasko TS, Wallen-Mackenzie A, Morales M, Rayport S, Sulzer D. The multilingual nature of dopamine neurons. Prog Brain Res. 2014;211:141–64.
Morales M, Margolis EB. Ventral tegmental area: cellular heterogeneity, connectivity and behaviour. Nat Rev Neurosci. 2017;18:73–85.
Buck SA, Erickson-Oberg MQ, Bhatte SH, McKellar CD, Ramanathan VP, Rubin SA, et al. Roles of VGLUT2 and dopamine/glutamate Co-transmission in selective vulnerability to dopamine neurodegeneration. ACS Chem Neurosci. 2022;13:187–93.
Mingote S, Amsellem A, Kempf A, Rayport S, Chuhma N. Dopamine-glutamate neuron projections to the nucleus accumbens medial shell and behavioral switching. Neurochemistry Int. 2019;129:104482.
Mingote S, Chuhma N, Kusnoor SV, Field B, Deutch AY, Rayport S. Functional connectome analysis of dopamine neuron glutamatergic connections in forebrain regions. J Neurosci. 2015;35:16259–71.
Chuhma N, Choi WY, Mingote S, Rayport S. Dopamine neuron glutamate cotransmission: frequency-dependent modulation in the mesoventromedial projection. Neuroscience. 2009;164:1068–83.
Stuber GD, Hnasko TS, Britt JP, Edwards RH, Bonci A. Dopaminergic terminals in the nucleus accumbens but not the dorsal striatum corelease glutamate. J Neurosci. 2010;30:8229–33.
Tecuapetla F, Patel JC, Xenias H, English D, Tadros I, Shah F, et al. Glutamatergic signaling by mesolimbic dopamine neurons in the nucleus accumbens. J Neurosci. 2010;30:7105–10.
Fortin GM, Ducrot C, Giguere N, Kouwenhoven WM, Bourque MJ, Pacelli C, et al. Segregation of dopamine and glutamate release sites in dopamine neuron axons: regulation by striatal target cells. FASEB J. 2019;33:400–17.
Silm K, Yang J, Marcott PF, Asensio CS, Eriksen J, Guthrie DA, et al. Synaptic vesicle recycling pathway determines neurotransmitter content and release properties. Neuron. 2019;102:786–800.e785.
Gulati M, Jain N, Davis JH, Williamson JR, Britton RA. Functional interaction between ribosomal protein L6 and RbgA during ribosome assembly. PLoS Genet. 2014;10:e1004694.
Yang C, Zang W, Ji Y, Li T, Yang Y, Zheng X. Ribosomal protein L6 (RPL6) is recruited to DNA damage sites in a poly(ADP-ribose) polymerase-dependent manner and regulates the DNA damage response. J Biol Chem. 2019;294:2827–38.
Wolf ME, LeWitt PA, Bannon MJ, Dragovic LJ, Kapatos G. Effect of aging on tyrosine hydroxylase protein content and the relative number of dopamine nerve terminals in human caudate. J Neurochem. 1991;56:1191–1200.
Srivastava P, Nishiyama S, Zhou F, Lin SH, Srivastava A, Su C, et al. Peripheral MC1R activation modulates immune responses and is neuroprotective in a mouse model of Parkinson’s disease. J Neuroimmune Pharmacol. 2023;18:704–17.
Cai W, Srivastava P, Feng D, Lin Y, Vanderburg CR, Xu Y, et al. Melanocortin 1 receptor activation protects against alpha-synuclein pathologies in models of Parkinson’s disease. Mol Neurodegener. 2022;17:16.
Di Lorenzo Alho AT, Suemoto CK, Polichiso L, Tampellini E, de Oliveira KC, Molina M, et al. Three-dimensional and stereological characterization of the human substantia nigra during aging. Brain Struct Funct. 2016;221:3393–403.
Pakkenberg H, Andersen BB, Burns RS, Pakkenberg B. A stereological study of substantia nigra in young and old rhesus monkeys. Brain Res. 1995;693:201–6.
Przedborski S, Levivier M, Jiang H, Ferreira M, Jackson-Lewis V, Donaldson D, et al. Dose-dependent lesions of the dopaminergic nigrostriatal pathway induced by intrastriatal injection of 6-hydroxydopamine. Neuroscience. 1995;67:631–47.
Meissner W, Prunier C, Guilloteau D, Chalon S, Gross CE, Bezard E. Time-course of nigrostriatal degeneration in a progressive MPTP-lesioned macaque model of Parkinson’s disease. Mol Neurobiol. 2003;28:209–18.
Bezard E, Dovero S, Prunier C, Ravenscroft P, Chalon S, Guilloteau D, et al. Relationship between the appearance of symptoms and the level of nigrostriatal degeneration in a progressive 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned macaque model of Parkinson’s disease. J Neurosci. 2001;21:6853–61.
Seniuk NA, Tatton WG, Greenwood CE. Dose-dependent destruction of the coeruleus-cortical and nigral-striatal projections by MPTP. Brain Res. 1990;527:7–20.
Sukoff Rizzo SJ, Anderson LC, Green TL, McGarr T, Wells G, Winter SS. Assessing healthspan and lifespan measures in aging mice: optimization of testing protocols, replicability, and rater reliability. Curr Protoc Mouse Biol. 2018;8:e45.
Dovonou A, Bolduc C, Soto Linan V, Gora C, Peralta Iii MR, Lévesque M. Animal models of Parkinson’s disease: bridging the gap between disease hallmarks and research questions. Transl Neurodegener. 2023;12:36.
Wolfarth S, Konieczny J, Smiałowska M, Schulze G, Ossowska K. Influence of 6-hydroxydopamine lesion of the dopaminergic nigrostriatal pathway on the muscle tone and electromyographic activity measured during passive movements. Neuroscience. 1996;74:985–96.
Olsson M, Nikkhah G, Bentlage C, Björklund A. Forelimb akinesia in the rat Parkinson model: differential effects of dopamine agonists and nigral transplants as assessed by a new stepping test. J Neurosci. 1995;15:3863–75.
Fleming SM, Zhu C, Fernagut PO, Mehta A, DiCarlo CD, Seaman RL, et al. Behavioral and immunohistochemical effects of chronic intravenous and subcutaneous infusions of varying doses of rotenone. Exp Neurol. 2004;187:418–29.
Guimarães RP, Resende MCS, Tavares MM, Belardinelli de Azevedo C, Ruiz MCM, Mortari MR. Construct, face, and predictive validity of Parkinson’s disease rodent models. Int J Mol Sci. 2024;25:8971.
Segovia G, Porras A, Del Arco A, Mora F. Glutamatergic neurotransmission in aging: a critical perspective. Mech Ageing Dev. 2001;122:1–29.
Dickstein DL, Kabaso D, Rocher AB, Luebke JI, Wearne SL, Hof PR. Changes in the structural complexity of the aged brain. Aging Cell. 2007;6:275–84.
Donzanti BA, Ung AK. Alterations in neurotransmitter amino acid content in the aging rat striatum are subregion dependent. Neurobiol Aging. 1990;11:159–62.
Benedetti MS, Russo A, Marrari P, Dostert P. Effects of ageing on the content in sulfur-containing amino acids in rat brain. J Neural Transm Gen Sect. 1991;86:191–203.
Corsi C, Melani A, Bianchi L, Pepeu G, Pedata F. Striatal A2A adenosine receptors differentially regulate spontaneous and K+-evoked glutamate release in vivo in young and aged rats. Neuroreport. 1999;10:687–91.
Segovia G, Del Arco A, Mora F. Effects of aging on the interaction between glutamate, dopamine, and GABA in striatum and nucleus accumbens of the awake rat. J Neurochem. 1999;73:2063–72.
Trudeau LE, El Mestikawy S. Glutamate cotransmission in cholinergic, GABAergic and monoamine systems: contrasts and commonalities. Front Neural Circuits. 2018;12:113.
Trudeau LE, Gutierrez R. On cotransmission & neurotransmitter phenotype plasticity. Mol Interv. 2007;7:138–46.
Hnasko TS, Edwards RH. Neurotransmitter corelease: mechanism and physiological role. Annu Rev Physiol. 2012;74:225–43.
Aguilar JI, Dunn M, Mingote S, Karam CS, Farino ZJ, Sonders MS, et al. Neuronal depolarization drives increased dopamine synaptic vesicle loading via VGLUT. Neuron. 2017;95:1074–1088.e1077.
Hnasko TS, Chuhma N, Zhang H, Goh GY, Sulzer D, Palmiter RD, et al. Vesicular glutamate transport promotes dopamine storage and glutamate corelease in vivo. Neuron. 2010;65:643–56.
Buck SA, Rubin SA, Kunkhyen T, Treiber CD, Xue X, Fenno LE, et al. Sexually dimorphic mechanisms of VGLUT-mediated protection from dopaminergic neurodegeneration. bioRxiv. 2023; https://doi.org/10.1101/2023.10.02.560584.
Kumar A, Thirumurugan K. Understanding cellular senescence: pathways involved, therapeutics and longevity aiding. Cell Cycle. 2023;22:1–22.
Shen R, Ardianto C, Celia C, Sidharta VM, Sasmita PK, Satriotomo I, et al. Brain-derived neurotrophic factor interplay with oxidative stress: neuropathology approach in potential biomarker of Alzheimer’s disease. Dement Neuropsychol. 2023;17:e20230012.
Lu T, Aron L, Zullo J, Pan Y, Kim H, Chen Y, et al. REST and stress resistance in ageing and Alzheimer’s disease. Nature. 2014;507:448–54.
Meiser J, Weindl D, Hiller K. Complexity of dopamine metabolism. Cell Commun Signal: CCS. 2013;11:34.
Van Laar VS, Chen J, Zharikov AD, Bai Q, Di Maio R, Dukes AA, et al. α-Synuclein amplifies cytoplasmic peroxide flux and oxidative stress provoked by mitochondrial inhibitors in CNS dopaminergic neurons in vivo. Redox Biol. 2020;37:101695.
Yang JH, Hayano M, Griffin PT, Amorim JA, Bonkowski MS, Apostolides JK, et al. Loss of epigenetic information as a cause of mammalian aging. Cell. 2023;186:305–326.e327.
Anirudhan A, Angulo-Bejarano PI, Paramasivam P, Manokaran K, Kamath SM, Murugesan R, et al. RPL6: a key molecule regulating zinc- and magnesium-bound metalloproteins of Parkinson’s disease. Front Neurosci. 2021;15:631892.
Dracheva S, Elhakem SL, McGurk SR, Davis KL, Haroutunian V. GAD67 and GAD65 mRNA and protein expression in cerebrocortical regions of elderly patients with schizophrenia. J Neurosci Res. 2004;76:581–92.
Di Maio A, De Rosa A, Pelucchi S, Garofalo M, Marciano B, Nuzzo T, et al. Analysis of mRNA and protein levels of CAP2, DLG1 and ADAM10 genes in post-mortem brain of schizophrenia, Parkinson’s and Alzheimer’s disease patients. Int J Mol Sci. 2022;23:1539.
Glausier JR, Lewis DA. Selective pyramidal cell reduction of GABA(A) receptor α1 subunit messenger RNA expression in schizophrenia. Neuropsychopharmacology. 2011;36:2103–10.
Zahn JM, Sonu R, Vogel H, Crane E, Mazan-Mamczarz K, Rabkin R, et al. Transcriptional profiling of aging in human muscle reveals a common aging signature. PLoS Genet. 2006;2:e115.
Davie K, Janssens J, Koldere D, De Waegeneer M, Pech U, Kreft Ł, et al. A single-cell transcriptome atlas of the aging drosophila brain. Cell. 2018;174:982–998.e920.
Parry TJ, Theisen JW, Hsu JY, Wang YL, Corcoran DL, Eustice M, et al. The TCT motif, a key component of an RNA polymerase II transcription system for the translational machinery. Genes Dev. 2010;24:2013–8.
Liu-Yesucevitz L, Bassell GJ, Gitler AD, Hart AC, Klann E, Richter JD, et al. Local RNA translation at the synapse and in disease. J Neurosci. 2011;31:16086–93.
Wang DO, Kim SM, Zhao Y, Hwang H, Miura SK, Sossin WS, et al. Synapse- and stimulus-specific local translation during long-term neuronal plasticity. Science. 2009;324:1536–40.
Heo S, Diering GH, Na CH, Nirujogi RS, Bachman JL, Pandey A, et al. Identification of long-lived synaptic proteins by proteomic analysis of synaptosome protein turnover. Proc Natl Acad Sci. 2018;115:E3827–e3836.
Calderwood SK, Murshid A, Prince T. The shock of aging: molecular chaperones and the heat shock response in longevity and aging-a mini-review. Gerontology. 2009;55:550–8.
David DC. Aging and the aggregating proteome. Front Genet. 2012;3:247.
Kaushik S, Cuervo AM. Proteostasis and aging. Nat Med. 2015;21:1406–15.
Labbadia J, Morimoto RI. Proteostasis and longevity: when does aging really begin? F1000Prime Rep. 2014;6:7.
Soti C, Csermely P. Aging and molecular chaperones. Exp Gerontol. 2003;38:1037–40.
Alegre-Abarrategui J, Brimblecombe KR, Roberts RF, Velentza-Almpani E, Tilley BS, Bengoa-Vergniory N, et al. Selective vulnerability in α-synucleinopathies. Acta Neuropathol. 2019;138:681–704.
Acknowledgements
We are grateful for discussions and technical assistance provided by Drs. Emma O’Leary, Tyler Fortuna, Eric Zimmerman, Stacey Sukoff Rizzo, and George Tseng. We thank Dr. Hriday Bhambhvani for statistical consultation and Hung-Ching (Rick) Chang for assistance with code deposition. Postmortem human brain tissue was provided by the NIH NeuroBioBank and the University of Pittsburgh Brain and Tissue Donation Program. This study was supported by The Pittsburgh Foundation (John F and Nancy A Emmerling Fund of the Pittsburgh Foundation, FPG00043 to ZF), the Commonwealth of Pennsylvania (PA-HEALTH to ZF), and the National Institutes of Health (R21AG068607 to ZF; R21AA028800 to ZF and RWL; R01ES034037 to ZF; 3R01ES034037-02A1 Supplement to ZF; R01DK124219 to ZF; R01DA061243 to ZF and RWL; F31NS11811 to SAB; R36DA057972 to JK; T32MH019986 to SJM; T32GM133353 to CW and JK).
Author information
Authors and Affiliations
Contributions
SAB and ZF conceived the project. SAB, SJM, JRG, CW, TBT, JK, KNF, and RWL performed imaging experiments and data analysis. JRG and DAL prepared postmortem human brain samples. CF and RWL performed the transcriptomic analyses. SAB, SJM, JK, and ZF wrote the manuscript with contributions from co-authors.
Corresponding author
Ethics declarations
Competing interests
ZF is funded by an investigator-initiated award from UPMC Enterprises, which is unrelated to the present study.
Ethics approval and consent to participate
All methods were performed in accordance with the relevant guidelines and regulations. All animal experiments were approved by the University of Pittsburgh Institutional Animal Care and Use Committee. For all research concerning human subjects, informed consent for donation was obtained from the decedent’s next-of-kin. All procedures were approved by the University of Pittsburgh’s Committee for the Oversight of Research and Clinical Training Involving Decedents and Institutional Review Board for Biomedical Research. None of the images in the current work are identifiable from human research participants.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Buck, S.A., Mabry, S.J., Glausier, J.R. et al. Aging disrupts the coordination between mRNA and protein expression in mouse and human midbrain. Mol Psychiatry 30, 3039–3054 (2025). https://doi.org/10.1038/s41380-025-02909-1
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41380-025-02909-1
This article is cited by
-
Impact of aging on the pharmacokinetic profile of everolimus in male mice
BMC Pharmacology and Toxicology (2026)