Abstract
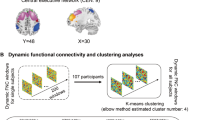
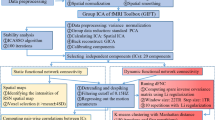
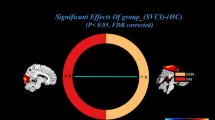
Obsessive-compulsive disorder (OCD) is signified by altered functional network connectivity (FNC), particularly within the default mode network (DMN), salience network (SAL), and fronto-parietal network (FPN). While previous studies suggest disruptions within triple networks, dynamic causal interactions across networks remain unaddressed. This study seeks to validate previous findings of static dysconnectivity between triple networks and further delineate the time-varying interactions and causal relationships among these networks in OCD. A resting-state functional magnetic resonance imaging study was performed on a relatively large and well-characterized clinical sample, comprising 88 medication-free OCD patients and 93 healthy controls (HC). Group independent component analysis, combined with a sliding window approach and k-means clustering analysis, was used to assess static and dynamic time-varying FNC within triple networks. Spectral dynamic causal modelling and parametric empirical Bayes framework were utilized to explore the abnormal effective connectivity among these networks in OCD patients. Our results proposed a novel dysregulated connectivity configuration of the triple-network model for OCD. With the self-inhibition increase in the left FPN, the excitatory effect onto the right FPN decrease, resulting in a weakened static connectivity between the left and right FPNs. Concurrently, time-varying hypoconnectivity patterns are observed between the left FPN and DMN, as well as the right FPN and SAL in OCD. Additionally, the excitatory influence from the DMN to the SAL suggests an atypical modulation within OCD’s network pathology. These findings advance our understanding of the dysregulated information transfer and the complex interplay of brain networks in OCD, potentially guiding future therapeutic strategies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
Abramowitz JS, Taylor S, McKay D. Obsessive-compulsive disorder. Lancet. 2009;374:491–9.
Stein DJ, Costa DLC, Lochner C, Miguel EC, Reddy YCL, Shavitt RG, et al. Obsessive-compulsive disorder. Nat Rev Dis Primers. 2019;5:52.
Benzina N, Mallet L, Burguière E, N’Diaye K, Pelissolo A. Cognitive dysfunction in obsessive-compulsive disorder. Curr Psychiatry Rep. 2016;18:80.
Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci. 2011;15:483–506.
Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci USA. 2008;105:12569–74.
Gürsel DA, Avram M, Sorg C, Brandl F, Koch K. Frontoparietal areas link impairments of large-scale intrinsic brain networks with aberrant fronto-striatal interactions in OCD: a meta-analysis of resting-state functional connectivity. Neurosci Biobehav Rev. 2018;87:151–60.
Bruin WB, Abe Y, Alonso P, Anticevic A, Backhausen LL, Balachander S, et al. The functional connectome in obsessive-compulsive disorder: resting-state mega-analysis and machine learning classification for the ENIGMA-OCD consortium. Mol Psychiatry. 2023;28:4320.
Tomiyama H, Murayama K, Nemoto K, Hasuzawa S, Mizobe T, Kato K, et al. Alterations of default mode and cingulo-opercular salience network and frontostriatal circuit: a candidate endophenotype of obsessive-compulsive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2022;116:110516.
Fan J, Zhong M, Gan J, Liu W, Niu C, Liao H, et al. Altered connectivity within and between the default mode, central executive, and salience networks in obsessive-compulsive disorder. J Affect Disord. 2017;223:106–14.
Fan J, Zhong M, Zhu X, Gan J, Liu W, Niu C, et al. Resting-state functional connectivity between right anterior insula and right orbital frontal cortex correlate with insight level in obsessive-compulsive disorder. Neuroimage Clin. 2017;15:1–7.
Beucke JC, Sepulcre J, Eldaief MC, Sebold M, Kathmann N, Kaufmann C. Default mode network subsystem alterations in obsessive-compulsive disorder. Br J Psychiatry. 2014;205:376–82.
Li P, Yang X, Greenshaw AJ, Li S, Luo J, Han H, et al. The effects of cognitive behavioral therapy on resting-state functional brain network in drug-naive patients with obsessive-compulsive disorder. Brain Behav. 2018;8:e00963.
Cyr M, Pagliaccio D, Yanes-Lukin P, Fontaine M, Rynn MA, Marsh R. Altered network connectivity predicts response to cognitive-behavioral therapy in pediatric obsessive-compulsive disorder. Neuropsychopharmacology. 2020;45:1232–40.
Shi TC, Pagliaccio D, Cyr M, Simpson HB, Marsh R. Network-based functional connectivity predicts response to exposure therapy in unmedicated adults with obsessive-compulsive disorder. Neuropsychopharmacology. 2021;46:1035–44.
Gürsel DA, Reinholz L, Bremer B, Schmitz-Koep B, Franzmeier N, Avram M, et al. Frontoparietal and salience network alterations in obsessive–compulsive disorder: insights from independent component and sliding time window analyses. J Psychiatry Neurosc. 2020;45:214–21.
Luo L, Li Q, You W, Wang Y, Tang W, Li B, et al. Altered brain functional network dynamics in obsessive-compulsive disorder. Hum Brain Mapp. 2021;42:2061–76.
Liu J, Li X, Xue K, Chen Y, Wang K, Niu Q, et al. Abnormal dynamics of functional connectivity in first-episode and treatment-naive patients with obsessive-compulsive disorder. Psychiatry Clin Neurosci. 2021;75:14–22.
Yan CG, Wang XD, Zuo XN, Zang YF. DPABI: data processing & analysis for (Resting-State) brain imaging. Neuroinformatics. 2016;14:339–51.
Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage. 2012;59:2142–54.
Supekar K, Cai W, Krishnadas R, Palaniyappan L, Menon V. Dysregulated brain dynamics in a triple-network saliency model of schizophrenia and its relation to psychosis. Biol Psychiatry. 2019;85:60–9.
Beckmann CF, Smith SM. Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE Trans Med Imaging. 2004;23:137–52.
Himberg J, Hyvärinen A, Esposito F. Validating the independent components of neuroimaging time series via clustering and visualization. NeuroImage. 2004;22:1214–22.
Kim J, Criaud M, Cho SS, Diez-Cirarda M, Mihaescu A, Coakeley S, et al. Abnormal intrinsic brain functional network dynamics in Parkinson’s disease. Brain. 2017;140:2955–67.
Fiorenzato E, Strafella AP, Kim J, Schifano R, Weis L, Antonini A, et al. Dynamic functional connectivity changes associated with dementia in Parkinson’s disease. Brain. 2019;142:2860–72.
Calhoun VD, Adali T, Pearlson GD, Pekar JJ. A method for making group inferences from functional MRI data using independent component analysis. Hum Brain Mapp. 2001;14:140–51.
Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106:1125–65.
Shirer WR, Ryali S, Rykhlevskaia E, Menon V, Greicius MD. Decoding subject-driven cognitive states with whole-brain connectivity patterns. Cereb Cortex. 2012;22:158–65.
Allen EA, Damaraju E, Plis SM, Erhardt EB, Eichele T, Calhoun VD. Tracking whole-brain connectivity dynamics in the resting state. Cereb Cortex. 2014;24:663–76.
Rashid B, Arbabshirani MR, Damaraju E, Cetin MS, Miller R, Pearlson GD, et al. Classification of schizophrenia and bipolar patients using static and dynamic resting-state fMRI brain connectivity. NeuroImage. 2016;134:645–57.
Preti MG, Bolton TA, Van De Ville D. The dynamic functional connectome: State-of-the-art and perspectives. NeuroImage. 2017;160:41–54.
Smith SM, Miller KL, Salimi-Khorshidi G, Webster M, Beckmann CF, Nichols TE, et al. Network modelling methods for FMRI. NeuroImage. 2011;54:875–91.
Friedman J, Hastie T, Tibshirani R. Sparse inverse covariance estimation with the graphical lasso. Biostatistics. 2008;9:432–41.
Aggarwal CC, Hinneburg A, Keim DA. On the surprising behavior of distance metrics in high dimensional space. In: Int. conf. on database theory 2001. LNCS vol 1973, pp 420–34. Springer; 2001.
Friston KJ, Kahan J, Biswal B, Razi A. A DCM for resting state fMRI. NeuroImage. 2014;94:396–407.
Zeidman P, Jafarian A, Corbin N, Seghier ML, Razi A, Price CJ, et al. A guide to group effective connectivity analysis, part 1: first level analysis with DCM for fMRI. NeuroImage. 2019;200:174–90.
Zeidman P, Jafarian A, Seghier ML, Litvak V, Cagnan H, Price CJ, et al. A guide to group effective connectivity analysis, part 2: second level analysis with PEB. NeuroImage. 2019;200:12–25.
Marchesi O, Bonacchi R, Valsasina P, Rocca MA, Filippi M. Resting state effective connectivity abnormalities of the Papez circuit and cognitive performance in multiple sclerosis. Mol Psychiatry. 2022;27:3913–9.
Ray D, Bezmaternykh D, Mel’nikov M, Friston KJ, Das M. Altered effective connectivity in sensorimotor cortices is a signature of severity and clinical course in depression. Proc Natl Acad Sci USA. 2021;118:e2105730118.
Thomas GEC, Zeidman P, Sultana T, Zarkali A, Razi A, Weil RS. Changes in both top-down and bottom-up effective connectivity drive visual hallucinations in Parkinson’s disease. Brain Commun. 2022;5:fcac329.
Friston KJ, Litvak V, Oswal A, Razi A, Stephan KE, van Wijk BCM, et al. Bayesian model reduction and empirical Bayes for group (DCM) studies. NeuroImage. 2016;128:413–31.
Penny WD, Stephan KE, Daunizeau J, Rosa MJ, Friston KJ, Schofield TM, et al. Comparing families of dynamic causal models. PLOS Comput Biol. 2010;6:e1000709.
Zhou Y, Friston KJ, Zeidman P, Chen J, Li S, Razi A. The hierarchical organization of the default, dorsal attention and salience networks in adolescents and young adults. Cereb Cortex. 2018;28:726–37.
Kass RE, Raftery AE. Bayes factors. J Am Stat Assoc. 1995;90:430.
Cole MW, Reynolds JR, Power JD, Repovs G, Anticevic A, Braver TS. Multi-task connectivity reveals flexible hubs for adaptive task control. Nat Neurosci. 2013;16:1348–55.
Marek S, Dosenbach NUF. The frontoparietal network: function, electrophysiology, and importance of individual precision mapping. Dialogues Clin Neurosci. 2018;20:133–40.
Dosenbach NUF, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RA, et al. Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci USA. 2007;104:11073–8.
Dosenbach NUF, Fair DA, Cohen AL, Schlaggar BL, Petersen SE. A dual-networks architecture of top-down control. Trends Cogn Sci. 2008;12:99–105.
Liu J, Cao L, Li H, Gao Y, Bu X, Liang K, et al. Abnormal resting-state functional connectivity in patients with obsessive-compulsive disorder: a systematic review and meta-analysis. Neurosci Biobehav Rev. 2022;135:104574.
Fornaro S, Vallesi A. Functional connectivity abnormalities of brain networks in obsessive–compulsive disorder: a systematic review. Curr Psychol. 2023;43:900–30.
Shephard E, Stern ER, van den Heuvel OA, Costa DLC, Batistuzzo MC, Godoy PBG, et al. Toward a neurocircuit-based taxonomy to guide treatment of obsessive-compulsive disorder. Mol Psychiatry. 2021;26:4583–604.
de Vries FE, de Wit SJ, Cath DC, van der Werf YD, van der Borden V, van Rossum TB, et al. Compensatory frontoparietal activity during working memory: an endophenotype of obsessive-compulsive disorder. Biol Psychiatry. 2014;76:878–87.
Gonçalves ÓF, Carvalho S, Leite J, Fernandes-Gonçalves A, Carracedo A, Sampaio A. Cognitive and emotional impairments in obsessive-compulsive disorder: evidence from functional brain alterations. Porto Biomed J. 2016;1:92–105.
Vaghi MM, Hampshire A, Fineberg NA, Kaser M, Bruhl AB, Sahakian BJ, et al. Hypoactivation and dysconnectivity of a frontostriatal circuit during goal-directed planning as an endophenotype for obsessive-compulsive disorder. Biol Psychiatry Cogn Neurosci Neuroimaging. 2017;2:655–63.
van den Heuvel OA, Veltman DJ, Groenewegen HJ, Cath DC, van Hartskamp J, Barkhof F, et al. Frontal-striatal dysfunction during planning in obsessive-compulsive disorder. Arch Gen Psychiatry. 2005;62:301–9.
Vaghi MM, Vértes PE, Kitzbichler MG, Apergis-Schoute AM, van der Flier FE, Fineberg NA, et al. Specific frontostriatal circuits for impaired cognitive flexibility and goal-directed planning in obsessive-compulsive disorder: evidence from resting-state functional connectivity. Biol Psychiatry. 2017;81:708–17.
Page LA, Rubia K, Deeley Q, Daly E, Toal F, Mataix-Cols D, et al. A functional magnetic resonance imaging study of inhibitory control in obsessive-compulsive disorder. Psychiatry Res. 2009;174:202–9.
Milad MR, Rauch SL. Obsessive-compulsive disorder: beyond segregated cortico-striatal pathways. Trends Cogn Sci. 2012;16:43–51.
Wang D, Buckner RL, Liu H. Functional specialization in the human brain estimated by intrinsic hemispheric interaction. J Neurosci. 2014;34:12341–52.
Chen AC, Oathes DJ, Chang C, Bradley T, Zhou ZW, Williams LM, et al. Causal interactions between fronto-parietal central executive and default-mode networks in humans. Proc Natl Acad Sci USA. 2013;110:19944–9.
Stern ER, Fitzgerald KD, Welsh RC, Abelson JL, Taylor SF. Resting-state functional connectivity between fronto-parietal and default mode networks in obsessive-compulsive disorder. PLoS One. 2012;7:e36356.
Liu Q, Gao F, Wang X, Xia J, Yuan G, Zheng S, et al. Cognitive inflexibility is linked to abnormal frontoparietal-related activation and connectivity in obsessive-compulsive disorder. Hum Brain Mapp. 2023;44:5460–70.
Chen YH, Li SF, Lv D, Zhu GD, Wang YH, Meng X, et al. Decreased intrinsic functional connectivity of the salience network in drug-naïve patients with obsessive-compulsive disorder. Front Neurosci. 2018;12:889.
Li H, Hu X, Gao Y, Cao L, Zhang L, Bu X, et al. Neural primacy of the dorsolateral prefrontal cortex in patients with obsessive-compulsive disorder. Neuroimage Clin. 2020;28:102432.
Xu Y, Han S, Wei Y, Zheng R, Cheng J, Zhang Y. Abnormal resting-state effective connectivity in large-scale networks among obsessive-compulsive disorder. Psychol Med. 2024;54:350–58.
Olatunji BO, Ferreira-Garcia R, Caseras X, Fullana MA, Wooderson S, Speckens A, et al. Predicting response to cognitive behavioral therapy in contamination-based obsessive-compulsive disorder from functional magnetic resonance imaging. Psychol Med. 2014;44:2125–37.
Liang K, Li H, Bu X, Li X, Cao L, Liu J, et al. Efficacy and tolerability of repetitive transcranial magnetic stimulation for the treatment of obsessive-compulsive disorder in adults: a systematic review and network meta-analysis. Transl Psychiatry. 2021;11:332.
Posner J, Song I, Lee S, Rodriguez CI, Moore H, Marsh R, et al. Increased functional connectivity between the default mode and salience networks in unmedicated adults with obsessive-compulsive disorder. Hum Brain Mapp. 2017;38:678–87.
Geffen T, Smallwood J, Finke C, Olbrich S, Sjoerds Z, Schlagenhauf F. Functional connectivity alterations between default mode network and occipital cortex in patients with obsessive-compulsive disorder (OCD). Neuroimage Clin. 2022;33:102915.
Xia J, Fan J, Liu W, Du H, Zhu J, Yi J, et al. Functional connectivity within the salience network differentiates autogenous- from reactive-type obsessive-compulsive disorder. Prog Neuro-psychopharmacol Biol Psychiatry. 2020;98:109813.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (Grant No. 82372080, 82402422), the Natural Science Foundation of Sichuan Province (Grant No. 2024NSFSC1558), the China Postdoctoral Science Foundation (Grant No. 2024M752241), the Postdoctor Research Fund of West China Hospital, Sichuan University (Grant No. 2024HXBH008), and the National Key R&D Program of China (Grant No. 2022YFF1202400).
Author information
Authors and Affiliations
Contributions
HL, BL, QG and XH designed research; HL, BL, LC, JJ, SC and HZ performed research; YG, LZ, XH and BB contributed analytic tools; HL, BL, ZZ and WB analyzed data; HL, BB and wrote the paper; QG, BB and XH reviewed the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, H., Li, B., Cao, L. et al. Dysregulated connectivity configuration of triple-network model in obsessive-compulsive disorder. Mol Psychiatry 30, 3138–3149 (2025). https://doi.org/10.1038/s41380-025-02921-5
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41380-025-02921-5
This article is cited by
-
Aberrant dynamics of the default mode network activity in patients with obsessive-compulsive disorder
Cognitive Neurodynamics (2025)