Abstract
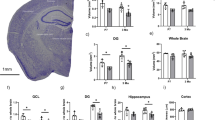
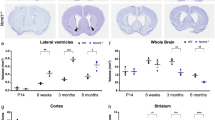
The Cre-lox site-specific recombinase system is one of the most powerful and versatile technology platforms for studying neural stem cells (NSCs) in adult brain, which is now challenged due to the complex and dynamic nature of in vivo gene expression. In this study, we develop an inducible dual recombinase-mediated intersectional genetics by combining Dre-rox and Cre-lox recombination technologies to specifically target two subpopulations of NSCs (α- and β-NSCs). By visiting their cell lineage and functionality, we find that α- and β-NSCs display distinct self-renewal and differentiation potential, as well as differential responses to external stimuli. Notably, in contrast to α-NSCs, the number of β-NSCs is not affected in aged mice and an APP/PS1 mouse model of Alzeimer’s disease. Single cell transcriptome analysis reveals divergent molecular signatures between type α- and β-NSCs and identifies PRMT1 as an important regulatory element to differentially regulate the neurogenic potential of α- and β-NSCs. Inhibition of PRMT1 specifically enhances the neurogenic capacity of β-NSCs and promotes the cognition functions in aged mice. Importantly, PRMT1 inhibition combined with increased BDNF levels pharmacologically ameliorates the cognitive impairments in APP/PS1 mice. Together, our study suggests that understanding the functional heterogeneity of NSCs might pave the way for harnessing the specific subpopulation of NSCs to treat brain disorders.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data generated in this study are included in this published article and its supplementary information files, which are available from the corresponding author on reasonable request. The raw scRNA-seq data in this study have been submitted to the Genome Sequence Archive in Beijing Institute of Genomics (BIG) Data Center (https://bigd.big.ac.cn/gsa) under accession number CRA012491.
References
Bond AM, Ming GL, Song H. Adult mammalian neural stem cells and neurogenesis: five decades later. Cell Stem Cell. 2015;17:385–95.
Li Y, Guo W. Neural stem cell niche and adult neurogenesis. Neuroscientist. 2021;27:235–45.
Toda T, Parylak SL, Linker SB, Gage FH. The role of adult hippocampal neurogenesis in brain health and disease. Mol Psychiatry. 2019;24:67–87.
Mu Y, Gage FH. Adult hippocampal neurogenesis and its role in Alzheimer’s disease. Mol Neurodegener. 2011;6:85.
Babcock KR, Page JS, Fallon JR, Webb AE. Adult hippocampal neurogenesis in aging and Alzheimer’s disease. Stem Cell Reports. 2021;16:681–93.
Moss J, Gebara E, Bushong EA, Sanchez-Pascual I, O’Laoi R, El M’Ghari I, et al. Fine processes of Nestin-GFP-positive radial glia-like stem cells in the adult dentate gyrus ensheathe local synapses and vasculature. Proc Natl Acad Sci USA. 2016;113:E2536–2545.
DeCarolis NA, Mechanic M, Petrik D, Carlton A, Ables JL, Malhotra S, et al. In vivo contribution of nestin- and GLAST-lineage cells to adult hippocampal neurogenesis. Hippocampus. 2013;23:708–19.
Luo X, Dai M, Wang M, Wang X, Guo W. Functional heterogeneity of Wnt-responsive and Hedgehog-responsive neural stem cells in the murine adult hippocampus. Dev Cell. 2023;58:2545–2562.e6.
Martin-Suarez S, Valero J, Muro-Garcia T, Encinas JM. Phenotypical and functional heterogeneity of neural stem cells in the aged hippocampus. Aging Cell. 2019;18:e12958.
Obernier K, Alvarez-Buylla A. Neural stem cells: origin, heterogeneity and regulation in the adult mammalian brain. Development. 2019;146:dev156059.
Otsuki L, Brand AH. Cell cycle heterogeneity directs the timing of neural stem cell activation from quiescence. Science. 2018;360:99–102.
Gebara E, Bonaguidi MA, Beckervordersandforth R, Sultan S, Udry F, Gijs PJ, et al. Heterogeneity of Radial Glia-Like Cells in the Adult Hippocampus. Stem Cells. 2016;34:997–1010.
Han X, Zhang Z, He L, Zhu H, Li Y, Pu W, et al. A suite of new Dre recombinase drivers markedly expands the ability to perform intersectional genetic targeting. Cell Stem Cell. 2021;28:1160–1176.e1167.
Madisen L, Garner AR, Shimaoka D, Chuong AS, Klapoetke NC, Li L, et al. Transgenic mice for intersectional targeting of neural sensors and effectors with high specificity and performance. Neuron. 2015;85:942–58.
Opendak M, Gould E. Adult neurogenesis: a substrate for experience-dependent change. Trends Cogn Sci. 2015;19:151–61.
Nilsson M, Perfilieva E, Johansson U, Orwar O, Eriksson PS. Enriched environment increases neurogenesis in the adult rat dentate gyrus and improves spatial memory. J Neurobiol. 1999;39:569–78.
Mich JK, Signer RA, Nakada D, Pineda A, Burgess RJ, Vue TY, et al. Prospective identification of functionally distinct stem cells and neurosphere-initiating cells in adult mouse forebrain. Elife. 2014;3:e02669.
Santello M, Toni N, Volterra A. Astrocyte function from information processing to cognition and cognitive impairment. Nat Neurosci. 2019;22:154–66.
Bergen V, Lange M, Peidli S, Wolf FA, Theis FJ. Generalizing RNA velocity to transient cell states through dynamical modeling. Nat Biotechnol. 2020;38:1408–14.
Ibrayeva A, Bay M, Pu E, Jorg DJ, Peng L, Jun H, et al. Early stem cell aging in the mature brain. Cell Stem Cell. 2021;28:955–966.e957.
Chen L, Zhang M, Fang L, Yang X, Cao N, Xu L, et al. Coordinated regulation of the ribosome and proteasome by PRMT1 in the maintenance of neural stemness in cancer cells and neural stem cells. J Biol Chem. 2021;297:101275.
Mudo G, Bonomo A, Di Liberto V, Frinchi M, Fuxe K, Belluardo N. The FGF-2/FGFRs neurotrophic system promotes neurogenesis in the adult brain. J Neural Transm (Vienna). 2009;116:995–1005.
Romano R, Bucci C. Role of EGFR in the nervous system. Cells. 2020;9:1887.
Schaffner I, Minakaki G, Khan MA, Balta EA, Schlotzer-Schrehardt U, Schwarz TJ, et al. FoxO function is essential for maintenance of autophagic flux and neuronal morphogenesis in adult neurogenesis. Neuron. 2018;99:1188–1203.e1186.
Zhang R, Boareto M, Engler A, Louvi A, Giachino C, Iber D, et al. Id4 downstream of Notch2 maintains neural stem cell quiescence in the adult hippocampus. Cell Rep. 2019;28:1485–1498.e1486.
Braun K, Haberle BM, Wittmann MT, Lie DC. Enriched environment ameliorates adult hippocampal neurogenesis deficits in Tcf4 haploinsufficient mice. BMC Neurosci. 2020;21:50.
Hashimoto M, Fukamizu A, Nakagawa T, Kizuka Y. Roles of protein arginine methyltransferase 1 (PRMT1) in brain development and disease. Biochim Biophys Acta Gen Subj. 2021;1865:129776.
Heinke R, Spannhoff A, Meier R, Trojer P, Bauer I, Jung M, et al. Virtual screening and biological characterization of novel histone arginine methyltransferase PRMT1 inhibitors. ChemMedChem. 2009;4:69–77.
Seri B, Garcia-Verdugo JM, McEwen BS, Alvarez-Buylla A. Astrocytes give rise to new neurons in the adult mammalian hippocampus. J Neurosci. 2001;21:7153–60.
Choi SH, Bylykbashi E, Chatila ZK, Lee SW, Pulli B, Clemenson GD, et al. Combined adult neurogenesis and BDNF mimic exercise effects on cognition in an Alzheimer’s mouse model. Science. 2018;361:eaan8821.
Crews L, Adame A, Patrick C, Delaney A, Pham E, Rockenstein E, et al. Increased BMP6 levels in the brains of Alzheimer’s disease patients and APP transgenic mice are accompanied by impaired neurogenesis. J Neurosci. 2010;30:12252–62.
Gomez-Nicola D, Suzzi S, Vargas-Caballero M, Fransen NL, Al-Malki H, Cebrian-Silla A, et al. Temporal dynamics of hippocampal neurogenesis in chronic neurodegeneration. Brain. 2014;137:2312–28.
Li B, Yamamori H, Tatebayashi Y, Shafit-Zagardo B, Tanimukai H, Chen S, et al. Failure of neuronal maturation in Alzheimer disease dentate gyrus. J Neuropathol Exp Neurol. 2008;67:78–84.
Lovell MA, Geiger H, Van Zant GE, Lynn BC, Markesbery WR. Isolation of neural precursor cells from Alzheimer’s disease and aged control postmortem brain. Neurobiol Aging. 2006;27:909–17.
Perry EK, Johnson M, Ekonomou A, Perry RH, Ballard C, Attems J. Neurogenic abnormalities in Alzheimer’s disease differ between stages of neurogenesis and are partly related to cholinergic pathology. Neurobiol Dis. 2012;47:155–62.
Tobin MK, Musaraca K, Disouky A, Shetti A, Bheri A, Honer WG, et al. Human hippocampal neurogenesis persists in aged adults and Alzheimer’s disease patients. Cell Stem Cell. 2019;24:974–982.e973.
Guerrieri D, van Praag H. Exercise-mimetic AICAR transiently benefits brain function. Oncotarget. 2015;6:18293–313.
Ganat YM, Silbereis J, Cave C, Ngu H, Anderson GM, Ohkubo Y, et al. Early postnatal astroglial cells produce multilineage precursors and neural stem cells in vivo. J Neurosci. 2006;26:8609–21.
Burns KA, Ayoub AE, Breunig JJ, Adhami F, Weng WL, Colbert MC, et al. Nestin-CreER mice reveal DNA synthesis by nonapoptotic neurons following cerebral ischemia hypoxia. Cereb Cortex. 2007;17:2585–92.
Furube E, Ishii H, Nambu Y, Kurganov E, Nagaoka S, Morita M, et al. Neural stem cell phenotype of tanycyte-like ependymal cells in the circumventricular organs and central canal of adult mouse brain. Sci Rep. 2020;10:2826.
Lagace DC, Whitman MC, Noonan MA, Ables JL, DeCarolis NA, Arguello AA, et al. Dynamic contribution of nestin-expressing stem cells to adult neurogenesis. J Neurosci. 2007;27:12623–9.
Hirrlinger J, Scheller A, Hirrlinger PG, Kellert B, Tang W, Wehr MC, et al. Split-cre complementation indicates coincident activity of different genes in vivo. PLoS One. 2009;4:e4286.
Kolodziejczyk AA, Kim JK, Svensson V, Marioni JC, Teichmann SA. The technology and biology of single-cell RNA sequencing. Mol Cell. 2015;58:610–20.
Dulken BW, Leeman DS, Boutet SC, Hebestreit K, Brunet A. Single-cell transcriptomic analysis defines heterogeneity and transcriptional dynamics in the adult neural stem cell lineage. Cell Rep. 2017;18:777–90.
Hao ZZ, Wei JR, Xiao D, Liu R, Xu N, Tang L, et al. Single-cell transcriptomics of adult macaque hippocampus reveals neural precursor cell populations. Nat Neurosci. 2022;25:805–17.
Hochgerner H, Zeisel A, Lonnerberg P, Linnarsson S. Conserved properties of dentate gyrus neurogenesis across postnatal development revealed by single-cell RNA sequencing. Nat Neurosci. 2018;21:290–9.
Llorens-Bobadilla E, Zhao S, Baser A, Saiz-Castro G, Zwadlo K, Martin-Villalba A. Single-cell transcriptomics reveals a population of dormant neural stem cells that become activated upon brain injury. Cell Stem Cell. 2015;17:329–40.
Mizrak D, Levitin HM, Delgado AC, Crotet V, Yuan J, Chaker Z, et al. Single-cell analysis of regional differences in adult V-SVZ neural stem cell lineages. Cell Rep. 2019;26:394–406 e395.
Shin J, Berg DA, Zhu Y, Shin JY, Song J, Bonaguidi MA, et al. Single-cell RNA-Seq with waterfall reveals molecular cascades underlying adult neurogenesis. Cell Stem Cell. 2015;17:360–72.
Wang W, Wang M, Yang M, Zeng B, Qiu W, Ma Q, et al. Transcriptome dynamics of hippocampal neurogenesis in macaques across the lifespan and aged humans. Cell Res. 2022;32:729–43.
Lahnemann D, Koster J, Szczurek E, McCarthy DJ, Hicks SC, Robinson MD, et al. Eleven grand challenges in single-cell data science. Genome Biol. 2020;21:31.
Thomsen R, Pallesen J, Daugaard TF, Borglum AD, Nielsen AL. Genome wide assessment of mRNA in astrocyte protrusions by direct RNA sequencing reveals mRNA localization for the intermediate filament protein nestin. Glia. 2013;61:1922–37.
Almazan G, Vela JM, Molina-Holgado E, Guaza C. Re-evaluation of nestin as a marker of oligodendrocyte lineage cells. Microsc Res Tech. 2001;52:753–65.
Takamori Y, Mori T, Wakabayashi T, Nagasaka Y, Matsuzaki T, Yamada H. Nestin-positive microglia in adult rat cerebral cortex. Brain Res. 2009;1270:10–18.
Mendez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA, et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466:829–34.
Jurkowski MP, Bettio L, K Woo E, Patten A, Yau SY, Gil-Mohapel J. Beyond the hippocampus and the SVZ: adult neurogenesis throughout the brain. Front Cell Neurosci. 2020;14:576444.
Gubert C, Hannan AJ. Exercise mimetics: harnessing the therapeutic effects of physical activity. Nat Rev Drug Discov. 2021;20:862–79.
He L, Li Y, Li Y, Pu W, Huang X, Tian X, et al. Enhancing the precision of genetic lineage tracing using dual recombinases. Nat Med. 2017;23:1488–98.
Tang C, Wang M, Wang P, Wang L, Wu Q, Guo W. Neural stem cells behave as a functional niche for the maturation of newborn neurons through the secretion of PTN. Neuron. 2019;101:32–44.e36.
Wang J, Cui Y, Yu Z, Wang W, Cheng X, Ji W, et al. Brain endothelial cells maintain lactate homeostasis and control adult hippocampal neurogenesis. Cell Stem Cell. 2019;25:754–767.e759.
Wang M, Tang C, Xing R, Liu X, Han X, Liu Y, et al. WDR81 regulates adult hippocampal neurogenesis through endosomal SARA-TGFbeta signaling. Mol Psychiatry. 2021;26:694–709.
Xu M, Guo Y, Wang M, Luo X, Shen X, Li Z, et al. L-arginine homeostasis governs adult neural stem cell activation by modulating energy metabolism in vivo. EMBO J. 2023;42:e112647.
Tang C, Guo W. Implantation of a mini-osmotic pump plus stereotactical injection of retrovirus to study newborn neuron development in adult mouse hippocampus. STAR Protoc. 2021;2:100374.
Funding
This research was supported by grants from STI2030-Major Projects (2021ZD0202302 to WG), the National Science Foundation of China (32394030 and 82271202 to WG).
Author information
Authors and Affiliations
Contributions
Ziqi Liang, Jinting He and Weixiang Guo conceived experiments; Ziqi Liang performed the analysis, created figures and drafted the manuscript. Zhimin Li, Jinting He, Jin Feng and Min Wang provided animals for the experiment, as well as interpretation of biological results. Xing Luo, Qiang Liu and Dezhe Qin performed the behavioral tests, as well as interpretation of biological results. Dan Zhang and Zhiheng Xu performed the single cell-RNA-seq analysis. Weixiang Guo wrote the manuscript. All authors read, revised, and approved the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This study did not involve any human subjects. All animal care and related experimental procedures were conducted following the highest ethical standards and were approved by the IGDB Institutional Animal Care and Use Committee. All animals were bred and raised by the Transgenic Mouse Facility at IGDB under a regular light/dark (12 h/12 h) cycle with ad libitum access to food and water.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liang, Z., Li, Z., Zhang, D. et al. Dual recombinase-mediated intersectional genetics defines the functional heterogeneity of neural stem cells in adult hippocampus. Mol Psychiatry 30, 3516–3532 (2025). https://doi.org/10.1038/s41380-025-02937-x
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41380-025-02937-x