Abstract
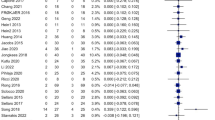
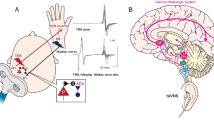
Negative symptoms in treatment-resistant schizophrenia (TRS) are notably persistent and minimally affected by antipsychotics, the transcutaneous auricular vagus nerve stimulation (taVNS) is a promising treatment approach. However, clinical trials are scarce, and further efficacy data are needed. We conducted a double-blind, sham-controlled, randomized clinical trial to determine the efficacy and safety of taVNS as an add-on treatment for patients with TRS with predominantly negative symptoms and to investigate potential biomarkers of efficacy. A total of 50 patients underwent a two-week intervention of active taVNS (n = 25) or sham taVNS (n = 25), followed by a two-week follow-up. Primary outcome was the change in the PANSS-factor score for negative symptoms (PANSS-FSNS) assessed after the intervention. In the intention-to-treat analysis, patients receiving active taVNS showed a significantly greater improvement in negative symptoms compared with those receiving the sham procedure (PANSS-FSNS difference, −1.36; effect size, −0.62; 95% CI, −1.20 to −0.04; p = 0.033), with effects sustained at follow-up and good tolerability. Inflammatory cytokines and EEG coherence showed that in the active group, the change in PANSS-FSNS scores after treatment was significantly correlated with changes in tumour necrosis factor (TNF)-α (r = 0.56, corrected p = 0.017) and beta-band coherence between the left frontal and parietal regions (r = −0.56, p = 0.004), but not in the sham group. This study suggests that taVNS may effectively and safely ameliorate negative symptoms in TRS, with TNF-α and beta-band coherence between the left frontal and parietal regions as potential sensitivity efficacy biomarkers. Chinese Clinical Trial Registry (http://www.chictr.org.cn.), ChiCTR2400085198.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
Study-related data are available from the corresponding author upon reasonable request and with approval from the hospital administration.
Code availability
The statistical analyses were conducted using R (version 4.2.3), and EEG feature extraction was performed using custom scripts written in MATLAB (version 2024a). The code is available from the corresponding author upon reasonable request.
References
Saha S, Chant D, Welham J, McGrath J, Farooq S, Choudry A, et al. A Systematic Review of the Prevalence of Schizophrenia. PLoS Med. 2005;2:e141.
Howes OD, McCutcheon R, Agid O, de Bartolomeis A, van Beveren NJM, Birnbaum ML, et al. Treatment-resistant schizophrenia: treatment response and resistance in psychosis (TRRIP) working group consensus guidelines on diagnosis and terminology. AJP. 2017;174:216–29.
Owen MJ, Sawa A, Mortensen PB. Schizophrenia. The Lancet. 2016;388:86–97.
Sicras-Mainar A, Maurino J, Ruiz-Beato E, Navarro-Artieda R. Impact of negative symptoms on healthcare resource utilization and associated costs in adult outpatients with schizophrenia: a population-based study. BMC Psychiatry. 2014;14:225.
Galderisi S, Mucci A, Buchanan RW, Arango C. Negative symptoms of schizophrenia: new developments and unanswered research questions. Lancet Psychiatry. 2018;5:664–77.
McCutcheon RA, Reis Marques T, Howes OD. Schizophrenia—An Overview. JAMA Psychiatry. 2020;77:201.
Fusar-Poli P, Papanastasiou E, Stahl D, Rocchetti M, Carpenter W, Shergill S, et al. Treatments of negative symptoms in schizophrenia: meta-analysis of 168 randomized placebo-controlled trials. Schizophr Bull. 2015;41:892–9.
Mishra BR, Agrawal K, Biswas T, Mohapatra D, Nath S, Maiti R. Comparison of acute followed by maintenance ECT vs clozapine on psychopathology and regional cerebral blood flow in treatment-resistant schizophrenia: a randomized controlled trial. Schizophr Bull. 2022;48:814–25.
Melzer-Ribeiro DL, Ribeiro Grilli-Tissot MC, Elkis H. ECT versus Sham for clozapine-resistant schizophrenia: A secondary analysis of a pilot study based on PANSS-30 individual items. Brain Stimul. 2020;13:1517–8.
Aleman A, Enriquez-Geppert S, Knegtering H, Dlabac-de Lange JJ. Moderate effects of noninvasive brain stimulation of the frontal cortex for improving negative symptoms in schizophrenia: Meta-analysis of controlled trials. Neurosci Biobehav Rev. 2018;89:111–8.
Tseng P-T, Zeng B-S, Hung C-M, Liang C-S, Stubbs B, Carvalho AF, et al. Assessment of noninvasive brain stimulation interventions for negative symptoms of schizophrenia: a systematic review and network meta-analysis. JAMA Psychiatry. 2022;79:770–9.
Lindenmayer JP, Kulsa MKC, Sultana T, Kaur A, Yang R, Ljuri I, et al. Transcranial direct-current stimulation in ultra-treatment-resistant schizophrenia. Brain Stimul. 2019;12:54–61.
Tuppurainen H, Määttä S, Könönen M, Julkunen P, Kautiainen H, Hyvärinen S, et al. Navigated and individual α-peak-frequency–guided transcranial magnetic stimulation in male patients with treatment-refractory schizophrenia. JPN. 2024;49:E87–E95.
Loo CK, Katalinic N, Smith DJ, Ingram A, Dowling N, Martin D, et al. A Randomized controlled trial of brief and ultrabrief pulse right unilateral electroconvulsive therapy. Int J Neuropsychopharmacol. 2014;18:pyu045.
Nettekoven C, Volz LJ, Leimbach M, Pool E-M, Rehme AK, Eickhoff SB, et al. Inter-individual variability in cortical excitability and motor network connectivity following multiple blocks of rTMS. Neuroimage. 2015;118:209.
Gianlorenco ACL, De Melo PS, Marduy A, Kim AY, Kim CK, Choi H, et al. Electroencephalographic Patterns in taVNS: A Systematic Review. Biomedicines. 2022;10:2208.
Gerez M, Tello A. Selected quantitative EEG (QEEG) and event-related potential (ERP) variables as discriminators for positive and negative schizophrenia. Biol Psychiatry. 1995;38:34–49.
Hudgens-Haney ME, Clementz BA, Ivleva EI, Keshavan MS, Pearlson GD, Gershon ES, et al. Cognitive impairment and diminished neural responses constitute a biomarker signature of negative symptoms in psychosis. Schizophr Bull. 2020;46:1269–81.
Sharaev MG, Malashenkova IK, Maslennikova AV, Zakharova NV, Bernstein AV, Burnaev EV, et al. Diagnosis of schizophrenia based on the data of various modalities: biomarkers and machine learning techniques (Review). Sovrem Tekhnologii Med. 2022;14:53–75.
Koopman FA, Chavan SS, Miljko S, Grazio S, Sokolovic S, Schuurman PR, et al. Vagus nerve stimulation inhibits cytokine production and attenuates disease severity in rheumatoid arthritis. Proc Natl Acad Sci USA. 2016;113:8284–9.
Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405:458–62.
Fox D. The shock tactics set to shake up immunology. Nature. 2017;545:20–22.
Lin A, Kenis G, Bignotti S, Tura G-J-B, De Jong R, Bosmans E, et al. The inflammatory response system in treatment-resistant schizophrenia: increased serum interleukin-6. Schizophr Res. 1998;32:9–15.
Goldsmith DR, Haroon E, Miller AH, Strauss GP, Buckley PF, Miller BJ. TNF-α and IL-6 are associated with the deficit syndrome and negative symptoms in patients with chronic schizophrenia. Schizophr Res. 2018;199:281.
Halstead S, Siskind D, Amft M, Wagner E, Yakimov V, Shih-Jung Liu Z, et al. Alteration patterns of peripheral concentrations of cytokines and associated inflammatory proteins in acute and chronic stages of schizophrenia: a systematic review and network meta-analysis. The Lancet Psychiatry. 2023;10:260–71.
Mondelli V, Ciufolini S, Belvederi Murri M, Bonaccorso S, Di Forti M, Giordano A, et al. Cortisol and inflammatory biomarkers predict poor treatment response in first episode psychosis. SCHBUL. 2015;41:1162–70.
Goldsmith DR, Rapaport MH. Inflammation and negative symptoms of Schizophrenia: implications for reward processing and motivational deficits. Front Psychiatry. 2020;11:46.
Goldsmith DR, Massa N, Pearce BD, Wommack EC, Alrohaibani A, Goel N, et al. Inflammatory markers are associated with psychomotor slowing in patients with schizophrenia compared to healthy controls. NPJ Schizophr. 2020;6:8.
Tan C, Qiao M, Ma Y, Luo Y, Fang J, Yang Y. The efficacy and safety of transcutaneous auricular vagus nerve stimulation in the treatment of depressive disorder: a systematic review and meta-analysis of randomized controlled trials. J Affect Disord. 2023;337:37–49.
Colle R, Ait Tayeb AEK, Delhay L, Boniface B, Gasnier M, Martin S, et al. Efficacy and safety of adjunctive vagus nerve stimulation in the treatment of resistant depression with psychotic features: a case report. Brain Stimul. 2021;14:498–9.
Wagner E, Kane JM, Correll CU, Howes O, Siskind D, Honer WG, et al. Clozapine combination and augmentation strategies in patients with schizophrenia —recommendations from an international expert survey among the treatment response and resistance in psychosis (TRRIP) working group. Schizophr Bull. 2020;46:1459–70.
Association WM. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310:2191–4.
Leucht S, Barabássy Á, Laszlovszky I, Szatmári B, Acsai K, Szalai E, et al. Linking PANSS negative symptom scores with the Clinical Global Impressions Scale: understanding negative symptom scores in schizophrenia. Neuropsychopharmacol. 2019;44:1589–96.
Addington D, Addington J, Maticka-tyndale E. Assessing depression in schizophrenia: the calgary depression scale. Br J Psychiatry. 1993;163:39–44.
Parente J, Carolyna Gianlorenco A, Rebello-Sanchez I, Kim M, Mario Prati J, Kyung Kim C, et al. Neural, anti-inflammatory, and clinical effects of transauricular vagus nerve stimulation in major depressive disorder: a systematic review. Int J Neuropsychopharmacol. 2023;27:pyad058.
Tian Q-Q, Cheng C, Yin Z-X, Yuan Y-Y, Wang C, Zeng X, et al. Combined transcutaneous auricular vagus stimulation (taVNS) with 0.1Hz slow breathing enhances insomnia treatment efficacy: A pilot study. Brain Stimul. 2024;17:4–6.
Sun J-B, Tian Q-Q, Yang X-J, Deng H, Li N, Meng L-X, et al. Synergistic effects of simultaneous transcranial direct current stimulation (tDCS) and transcutaneous auricular vagus nerve stimulation (taVNS) on the brain responses. Brain Stimul. 2021;14:417–9.
Shi X, Zhao L, Luo H, Deng H, Wang X, Ren G, et al. Transcutaneous auricular vagal nerve stimulation is effective for the treatment of functional dyspepsia: a multicenter, randomized controlled study. Am J Gastroenterol. 2024;119:521–31.
Sun J-B, Cheng C, Tian Q-Q, Yuan H, Yang X-J, Deng H, et al. Transcutaneous auricular vagus nerve stimulation improves spatial working memory in healthy young adults. Front Neurosci. 2021;15:790793.
Shen L-L, Sun J-B, Yang X-J, Deng H, Qin W, Du M-Y, et al. Reassessment of the effect of transcutaneous auricular vagus nerve stimulation using a novel burst paradigm on cardiac autonomic function in healthy young adults. Neuromodulation. 2022;25:433–42.
Zhao R, He Z-Y, Cheng C, Tian Q-Q, Cui Y-P, Chang M-Y, et al. Assessing the effect of simultaneous combining of transcranial direct current stimulation and transcutaneous auricular vagus nerve stimulation on the improvement of working memory performance in healthy individuals. Front Neurosci. 2022;16:947236.
Zhao R, Chang M-Y, Cheng C, Tian Q-Q, Yang X-J, Du M-Y, et al. Transcutaneous auricular vagus stimulation (taVNS) improves human working memory performance under sleep deprivation stress. Behav Brain Res. 2023;439:114247.
Tian Q-Q, Cheng C, Liu P-H, Yin Z-X, Zhang M-K, Cui Y-P, et al. Combined effect of transcutaneous auricular vagus nerve stimulation and 0.1 Hz slow-paced breathing on working memory. Front Neurosci. 2023;17:1133964.
Evensen K, Jørgensen MB, Sabers A, Martiny K. Transcutaneous vagal nerve stimulation in treatment-resistant depression: a feasibility study. Neuromodulation. 2022;25:443–9.
Kaczmarczyk M, Antosik-Wójcińska A, Dominiak M, Święcicki. Use of transcutaneous auricular vagus nerve stimulation (taVNS) in the treatment of drug-resistant depression - a pilot study, presentation of five clinical cases. Psychiatr Pol. 2021;55:555–64.
Frangos E, Ellrich J, Komisaruk BR. Non-invasive access to the vagus nerve central projections via electrical stimulation of the external ear: fMRI evidence in humans. Brain Stimul. 2015;8:624–36.
Andreasen NC. The Scale for the Assessment of Negative Symptoms (SANS): conceptual and theoretical foundations. Br J Psychiatry Suppl. 1989;4:49–58.
Pinna F, Deriu L, Diana E, Perra V, Randaccio RP, Sanna L, et al. Clinical Global Impression-severity score as a reliable measure for routine evaluation of remission in schizophrenia and schizoaffective disorders. Ann Gen Psychiatry. 2015;14:6.
Addington D, Addington J, Matickatyndale E. Specificity of the calgary depression scale for schizophrenics. Schizophr Res. 1994;11:239–44.
Wobrock T, Guse B, Cordes J, Wölwer W, Winterer G, Gaebel W, et al. Left Prefrontal high-frequency repetitive transcranial magnetic stimulation for the treatment of schizophrenia with predominant negative symptoms: a sham-controlled, randomized multicenter trial. Biol Psychiatry. 2015;77:979–88.
Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004;134:9–21.
Faul F, Erdfelder E, Lang A-G, Buchner A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175–91.
Niknian M. Permutation tests: a practical guide to resampling methods for testing hypotheses. Technometrics. 1995;37:341–2.
Ludbrook J, Dudley H. Why permutation tests are superior to t and F tests in biomedical research. Am Stat. 1998;52:127–32.
Buuren SV, Groothuis-Oudshoorn K. mice: Multivariate Imputation by Chained Equations in R. J Stat Soft. 2011;45:1–67.
Pesarin F, Salmaso L. Permutation Tests for Complex Data: Theory, Applications and Software. John Wiley & Sons; 2010.
Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing - Benjamini - 1995 - Journal of the Royal Statistical Society: Series B (Methodological) - Wiley Online Library. https://rss.onlinelibrary.wiley.com/doi/abs/10.1111/j.2517-6161.1995.tb02031.x. Accessed 10 November 2024.
Leucht S, Priller J, Davis JM. Antipsychotic drugs: a concise review of history, classification, indications, mechanism, efficacy, side effects, dosing, and clinical application. Am J Psychiatry. 2024;181:865–78.
Hasan A, Wolff-Menzler C, Pfeiffer S, Falkai P, Weidinger E, Jobst A, et al. Transcutaneous noninvasive vagus nerve stimulation (tVNS) in the treatment of schizophrenia: a bicentric randomized controlled pilot study. Eur Arch Psychiatry Clin Neurosci. 2015;265:589–600.
Kirkpatrick B, Fenton WS, Carpenter WT, Marder SR. The NIMH-MATRICS consensus statement on negative symptoms. Schizophr Bull. 2006;32:214–9.
Furukawa TA, Levine SZ, Tanaka S, Goldberg Y, Samara M, Davis JM, et al. Initial severity of schizophrenia and efficacy of antipsychotics: participant-level meta-analysis of 6 placebo-controlled studies. JAMA Psychiatry. 2015;72:14.
Lieslehto J, Tiihonen J, Lähteenvuo M, Tanskanen A, Taipale H. Primary nonadherence to antipsychotic treatment among persons with schizophrenia. Schizophr Bull. 2022;48:655–63.
Perez SM, Carreno FR, Frazer A, Lodge DJ. Vagal nerve stimulation reverses aberrant dopamine system function in the methylazoxymethanol acetate rodent model of schizophrenia. J Neurosci. 2014;34:9261–7.
Kane JM. Tools to assess negative symptoms in schizophrenia. J Clin Psychiatry. 2013;74:e12.
Rabany L, Weiser M, Werbeloff N, Levkovitz Y. Assessment of negative symptoms and depression in schizophrenia: revision of the SANS and how it relates to the PANSS and CDSS. Schizophr Res. 2011;126:226–30.
Marder SR, Kirkpatrick B. Defining and measuring negative symptoms of schizophrenia in clinical trials. Eur Neuropsychopharmacol. 2014;24:737–43.
Chang C-C, Kao Y-C, Chao C-Y, Tzeng N-S, Chang H-A, Corripio I, et al. Deep brain stimulation in treatment resistant schizophrenia: a pilot randomized cross-over clinical trial. EBioMedicine. 2020;51:102568.
Kayo M, Scemes S, Savoia MG, Bichuette A, Abreu AC, Da Silva EP, et al. A randomized controlled trial of social skills training for patients with treatment-resistant schizophrenia with predominantly negative symptoms. Psychiatry Res. 2020;287:112914.
Valiengo LDCL, Goerigk S, Gordon PC, Padberg F, Serpa MH, Koebe S, et al. Efficacy and safety of transcranial direct current stimulation for treating negative symptoms in schizophrenia. JAMA Psychiatry. 2020;77:121–9.
Howes O, Fusar-Poli P, Osugo M. Treating negative symptoms of schizophrenia: current approaches and future perspectives. Br J Psychiatry. 2023;223:332–5.
Salazar de Pablo G, Besana F, Arienti V, Catalan A, Vaquerizo-Serrano J, Cabras A, et al. Longitudinal outcome of attenuated positive symptoms, negative symptoms, functioning and remission in people at clinical high risk for psychosis: a meta-analysis. EClinicalMedicine. 2021;36:100909.
Huston JM, Ochani M, Rosas-Ballina M, Liao H, Ochani K, Pavlov VA, et al. Splenectomy inactivates the cholinergic antiinflammatory pathway during lethal endotoxemia and polymicrobial sepsis. J Exp Med. 2006;203:1623–8.
Rosas-Ballina M, Ochani M, Parrish WR, Ochani K, Harris YT, Huston JM, et al. Splenic nerve is required for cholinergic antiinflammatory pathway control of TNF in endotoxemia. Proc Natl Acad Sci USA. 2008;105:11008–13.
Olofsson PS, Levine YA, Caravaca A, Chavan SS, Pavlov VA, Faltys M, et al. Single-pulse and unidirectional electrical activation of the cervical vagus nerve reduces tumor necrosis factor in endotoxemia. Bioelectron Med. 2015;2:37–42.
Goldsmith DR, Bekhbat M, Mehta ND, Felger JC. Inflammation-related functional and structural dysconnectivity as a pathway to psychopathology. Biol Psychiatry. 2023;93:405–18.
Krynicki CR, Dazzan P, Pariante CM, Barnes NM, Vincent RC, Roberts A, et al. Deconstructing depression and negative symptoms of schizophrenia; differential and longitudinal immune correlates, and response to minocycline treatment. Brain Behav Immun. 2021;91:498–504.
Miller AH, Haroon E, Felger JC. Therapeutic implications of brain-immune interactions: treatment in translation. Neuropsychopharmacology. 2017;42:334–59.
Ogyu K, Matsushita K, Honda S, Wada M, Tamura S, Takenouchi K, et al. Decrease in gamma-band auditory steady-state response in patients with treatment-resistant schizophrenia. Schizophr Res. 2023;252:129–37.
Nucifora FC, Woznica E, Lee BJ, Cascella N, Sawa A. Treatment resistant schizophrenia: Clinical, biological, and therapeutic perspectives. Neurobiol Dis. 2019;131:104257.
Molent C, Olivo D, Wolf RC, Balestrieri M, Sambataro F. Functional neuroimaging in treatment resistant schizophrenia: a systematic review. Neurosci Biobehav Rev. 2019;104:178–90.
Takahashi T, Goto T, Nobukawa S, Tanaka Y, Kikuchi M, Higashima M, et al. Abnormal functional connectivity of high-frequency rhythms in drug-naïve schizophrenia. Clin Neurophysiol. 2018;129:222–31.
MacKay M-AB, Paylor JW, Wong JTF, Winship IR, Baker GB, Dursun SM. Multidimensional connectomics and treatment-resistant schizophrenia: linking phenotypic circuits to targeted therapeutics. Front Psychiatry. 2018;9:537.
Higashima M, Takeda T, Kikuchi M, Nagasawa T, Koshino Y. Functional connectivity between hemispheres and schizophrenic symptoms: a longitudinal study of interhemispheric EEG coherence in patients with acute exacerbations of schizophrenia. Clin EEG Neurosci. 2006;37:10–15.
Keatch C, Lambert E, Kameneva T, Woods W. Functional connectivity analysis of transcutaneous vagus nerve stimulation (tVNS) using magnetoencephalography (MEG). IEEE Trans Neural Syst Rehabil Eng. 2023;31:3630–40.
Zhang Y, Lin P, Wang R, Zhou J, Xu X, Jiang W, et al. Insula-medial prefrontal cortex functional connectivity modulated by transcutaneous auricular vagus nerve stimulation: an fMRI study. IEEE J Biomed Health Inform. 2024;28:5962–70.
Poppa T, Benschop L, Horczak P, Vanderhasselt M-A, Carrette E, Bechara A, et al. Auricular transcutaneous vagus nerve stimulation modulates the heart-evoked potential. Brain Stimul. 2022;15:260–9.
Engelen T, Solcà M, Tallon-Baudry C. Interoceptive rhythms in the brain. Nat Neurosci. 2023;26:1670–84.
Ferstl M, Teckentrup V, Lin WM, Kräutlein F, Kühnel A, Klaus J, et al. Non-invasive vagus nerve stimulation boosts mood recovery after effort exertion. Psychol Med. 2022;52:3029–39.
Ventura-Bort C, Weymar M. Transcutaneous auricular vagus nerve stimulation modulates the processing of interoceptive prediction error signals and their role in allostatic regulation. Hum Brain Mapp. 2024;45:e26613.
Kraus T, Kiess O, Hösl K, Terekhin P, Kornhuber J, Forster C. CNS BOLD fMRI effects of sham-controlled transcutaneous electrical nerve stimulation in the left outer auditory canal – a pilot study. Brain Stimul. 2013;6:798–804.
Andreasen NC, O’Leary DS, Flaum M, Nopoulos P, Watkins GL, Ponto LLB, et al. Hypofrontality in schizophrenia: distributed dysfunctional circuits in neuroleptic-naïve patients. The Lancet. 1997;349:1730–4.
Shukla DK, Chiappelli JJ, Sampath H, Kochunov P, Hare SM, Wisner K, et al. Aberrant frontostriatal connectivity in negative symptoms of schizophrenia. Schizophr Bull. 2019;45:1051–9.
Sheffield JM, Huang AS, Rogers BP, Blackford JU, Heckers S, Woodward ND. Insula sub-regions across the psychosis spectrum: morphology and clinical correlates. Transl Psychiatry. 2021;11:346.
Burke MJ, Kaptchuk TJ, Pascual-Leone A. Challenges of differential placebo effects in contemporary medicine: the example of brain stimulation. Ann Neurol. 2019;85:12–20.
Acknowledgements
The present study was partly funded by the Xidian University Specially Funded Project for Interdisciplinary Exploration (grant number TZJH2024014), which is gratefully acknowledged. We thank all research assistants, physicians, and nursing staffs at Kunming Psychiatric Hospital for their assistance during the study process, as well as the technicians in the EEG room, who performed the EEG collection, without whom this work could have been possible. We are also grateful to Yixuan Wu, Yu Tao and Hao Jing for their valuable support during the early stages of the project.
Author information
Authors and Affiliations
Contributions
JB.S, Y.G, and W.Q contributed to conceptualization and methodology; BK.Z, TP.G, SM.Z, ZQ.L, Y.C, MB.S, DNY.W, JX.W, and Q.W contributed to collect the clinical data or implement the interventions; JN.W, L.W, and XH.L contributed to collate the clinical data; YP.C, YY.Y, and F.H contributed to formal analysis and visualization of clinical trial data; QQ.T contributed to write the intervention program; YP.C and F.H contributed to writing the original draft; JB.S contributed to revised drafts. All authors contributed to review and editing of the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
This study was performed in accordance with the Declaration of Helsinki and was approved by the Kunming Psychiatric Hospital in China Review Board. Informed consent was provided by all of the participants.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cui, Y., Sun, J., Zhang, B. et al. Efficacy and safety of transcutaneous auricular vagus nerve stimulation for patients with treatment-resistant schizophrenia with predominantly negative symptoms: a randomized clinical trial and efficacy sensitivity biomarkers. Mol Psychiatry 30, 5437–5447 (2025). https://doi.org/10.1038/s41380-025-03132-8
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41380-025-03132-8