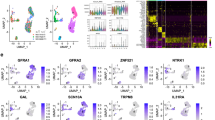
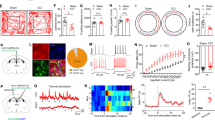
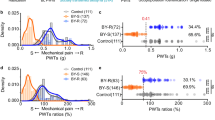
Abstract
Stress impacts pain sensation and its development, but the underlying neural mechanisms are largely unclear. Using restraint stress models and complete Freund’s adjuvant-induced pain model in male mice, we demonstrated that acute restraint stress (ARS) induces analgesia in both naïve and pain states. In contrast, chronic restraint stress (CRS) enhances pain hypersensitivity in naïve states, prolongs pain duration, and promotes anxiodepressive symptoms in pain states. Notably, ARS and CRS distinctly activate neuronal ensembles in the paraventricular nucleus of the hypothalamus (PVN). Using the targeted recombination in active populations strategy and chemogenetics, we found that these neuronal ensembles mediate the effects of acute and chronic stress on pain sensation and development. Furthermore, through a two-vector strategy and chemogenetic approach, these neuronal ensembles appear to exert their effects via PVN-locus coeruleus and PVN-lateral septum projections, respectively. Overall, our findings offer novel insights into pain sensation and pain chronification, and may provide effective therapeutic strategies for clinical pain and emotional comorbidities.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data to support the conclusions are present in the paper or supplementary materials. Because the raw data are huge and presented in diverse formats, the raw data are available from the corresponding author upon request.
References
Dahlhamer J, Lucas J, Zelaya C, Nahin R, Mackey S, DeBar L, et al. Prevalence of chronic pain and high-impact chronic pain among adults - United States, 2016. MMWR Morb Mortal Wkly Rep. 2018;67:1001–6.
Dudeney J, Aaron RV, Hathway T, Bhattiprolu K, Bisby MA, McGill LS, et al. Anxiety and depression in youth with chronic pain: a systematic review and meta-analysis. JAMA Pediatr. 2024;178:1114–23.
Wiech K, Tracey I. The influence of negative emotions on pain: behavioral effects and neural mechanisms. Neuroimage. 2009;47:987–94.
Butler RK, Finn DP. Stress-induced analgesia. Prog Neurobiol. 2009;88:184–202.
Sorge RE, Martin LJ, Isbester KA, Sotocinal SG, Rosen S, Tuttle AH, et al. Olfactory exposure to males, including men, causes stress and related analgesia in rodents. Nat Methods. 2014;11:629–32.
Jennings EM, Okine BN, Roche M, Finn DP. Stress-induced hyperalgesia. Prog Neurobiol. 2014;121:1–18.
Vachon-Presseau E. Effects of stress on the corticolimbic system: implications for chronic pain. Prog Neuropsychopharmacol Biol Psychiatry. 2018;87:216–23.
Timmers I, Quaedflieg CWEM, Hsu C, Heathcote LC, Rovnaghi CR, Simons LE. The interaction between stress and chronic pain through the lens of threat learning. Neurosci Biobehav Rev. 2019;107:641–55.
Vidal C, Girault JM, Jacob J. The effect of pituitary removal on pain regulation in the rat. Brain Res. 1982;233:53–64.
Bodnar RJ, Glusman M, Brutus M, Spiaggia A, Kelly DD. Analgesia induced by cold-water stress: attenuation following hypophysectomy. Physiol Behav. 1979;23:53–62.
Hu S-W, Zhang Q, Xia S-H, Zhao W-N, Li Q-Z, Yang J-X, et al. Contralateral projection of anterior cingulate cortex contributes to mirror-image pain. J Neurosci. 2021;41:9988–10003.
Root CM, Denny CA, Hen R, Axel R. The participation of cortical amygdala in innate, odour-driven behaviour. Nature. 2014;515:269–73.
Wang D, Pan X, Zhou Y, Wu Z, Ren K, Liu H, et al. Lateral septum-lateral hypothalamus circuit dysfunction in comorbid pain and anxiety. Mol Psychiatry. 2023;28:1090–1100.
Zhang X, He T, Wu Z, Wang Y, Liu H, Zhang B, et al. The role of CD38 in inflammation-induced depression-like behavior and the antidepressant effect of (R)-ketamine. Brain Behav Immun. 2024;115:64–79.
Guenthner CJ, Miyamichi K, Yang HH, Heller HC, Luo L. Permanent genetic access to transiently active neurons via TRAP: targeted recombination in active populations. Neuron. 2013;78:773–84.
Jeong M, Choi J-H, Jang H, Sohn DH, Wang Q, Lee J, et al. Viral vector-mediated transgene delivery with novel recombinase systems for targeting neuronal populations defined by multiple features. Neuron. 2024;112:56–72.e54.
Xu Z, Hu S-W, Zhou Y, Guo Q, Wang D, Gao Y-H, et al. Corticotropin-releasing factor neurones in the paraventricular nucleus of the hypothalamus modulate isoflurane anaesthesia and its responses to acute stress in mice. Br J Anaesth. 2023;130:446–58.
Xiong F, Yang H, Song Y-G, Qin H-B, Zhang Q-Y, Huang X, et al. An HSV-1-H129 amplicon tracer system for rapid and efficient monosynaptic anterograde neural circuit tracing. Nat Commun. 2022;13:7645.
Lee H-J, Chang L-Y, Ho Y-C, Teng S-F, Hwang L-L, Mackie K, et al. Stress induces analgesia via orexin 1 receptor-initiated endocannabinoid/CB1 signaling in the mouse periaqueductal gray. Neuropharmacology. 2016;105:577–86.
Li J, Sha L, Xu Q. An early increase in glutamate is critical for the development of depression-like behavior in a chronic restraint stress (CRS) model. Brain Res Bull. 2020;162:59–66.
Pan Z, Zhang Q, Liu X, Zhou H, Jin T, Hao L-Y, et al. Methyltransferase-like 3 contributes to inflammatory pain by targeting TET1 in YTHDF2-dependent manner. Pain. 2021;162:1960–76.
Zhang S, Yang X-N, Zang T, Luo J, Pan Z, Wang L, et al. Astroglial MicroRNA-219-5p in the ventral tegmental area regulates nociception in rats. Anesthesiology. 2017;127:548–64.
Wolf G, Yirmiya R, Kreisel T, Goshen I, Weidenfeld J, Poole S, et al. Interleukin-1 signaling modulates stress-induced analgesia. Brain Behav Immun. 2007;21:652–9.
Abdelhamid RE, Kovacs KJ, Pasley JD, Nunez MG, Larson AA. Forced swim-induced musculoskeletal hyperalgesia is mediated by CRF2 receptors but not by TRPV1 receptors. Neuropharmacology. 2013;72:29–37.
Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci. 2009;10:397–409.
Ferguson AV, Latchford KJ, Samson WK. The paraventricular nucleus of the hypothalamus - a potential target for integrative treatment of autonomic dysfunction. Expert Opin Ther Targets. 2008;12:717–27.
Sawchenko PE, Swanson LW, Vale WW. Corticotropin-releasing factor: co-expression within distinct subsets of oxytocin-, vasopressin-, and neurotensin-immunoreactive neurons in the hypothalamus of the male rat. J Neurosci. 1984;4:1118–29.
Liu Y, Li A, Bair-Marshall C, Xu H, Jee HJ, Zhu E, et al. Oxytocin promotes prefrontal population activity via the PVN-PFC pathway to regulate pain. Neuron. 2023;111:1795–811.e1797.
Eliava M, Melchior M, Knobloch-Bollmann HS, Wahis J, da Silva Gouveia M, Tang Y, et al. A new population of parvocellular oxytocin neurons controlling magnocellular neuron activity and inflammatory pain processing. Neuron. 2016;89:1291–304.
Li Y-C, Zhang F-C, Li D, Weng R-X, Yu Y, Gao R, et al. Distinct circuits and molecular targets of the paraventricular hypothalamus decode visceral and somatic pain. Neuron. 2024;112:3734–49.e3735.
Li Y-J, Du W-J, Liu R, Zan G-Y, Ye B-L, Li Q, et al. Paraventricular nucleus-central amygdala oxytocinergic projection modulates pain-related anxiety-like behaviors in mice. CNS Neurosci Ther. 2023;29:3493–506.
Chang X, Zhang H, Chen S. Neural circuits regulating visceral pain. Commun Biol. 2024;7:457.
Suárez-Pereira I, Llorca-Torralba M, Bravo L, Camarena-Delgado C, Soriano-Mas C, Berrocoso E. The role of the locus coeruleus in pain and associated stress-related disorders. Biol Psychiatry. 2022;91:786–97.
Atzori M, Cuevas-Olguin R, Esquivel-Rendon E, Garcia-Oscos F, Salgado-Delgado RC, Saderi N, et al. Locus ceruleus norepinephrine release: a central regulator of CNS spatio-temporal activation? Front Synaptic Neurosci. 2016;8:25.
Menon R, Süß T, Oliveira VEM, Neumann ID, Bludau A. Neurobiology of the lateral septum: regulation of social behavior. Trends Neurosci. 2022;45:27–40.
Li Y-C, Wang Q, Li M-G, Hu S-F, Xu G-Y. A paraventricular hypothalamic nucleus input to ventral of lateral septal nucleus controls chronic visceral pain. Pain. 2023;164:625–37.
Xu Y, Lu Y, Cassidy RM, Mangieri LR, Zhu C, Huang X, et al. Identification of a neurocircuit underlying regulation of feeding by stress-related emotional responses. Nat Commun. 2019;10:3446.
Chapman CR, Tuckett RP, Song CW. Pain and stress in a systems perspective: reciprocal neural, endocrine, and immune interactions. J Pain. 2008;9:122–45.
Ahmad AH, Zakaria R. Pain in times of stress. Malays J Med Sci. 2015;22:52–61.
Heim C, Newport DJ, Mletzko T, Miller AH, Nemeroff CB. The link between childhood trauma and depression: insights from HPA axis studies in humans. Psychoneuroendocrinology. 2008;33:693–710.
Grau JW, Hyson RL, Maier SF, Madden J, Barchas JD. Long-term stress-induced analgesia and activation of the opiate system. Science. 1981;213:1409–11.
Hohmann AG, Suplita RL, Bolton NM, Neely MH, Fegley D, Mangieri R, et al. An endocannabinoid mechanism for stress-induced analgesia. Nature. 2005;435:1108–12.
Lee MT, Chiu Y-T, Chiu Y-C, Hor CC, Lee H-J, Guerrini R, et al. Neuropeptide S-initiated sequential cascade mediated by OX1, NK1, mGlu5 and CB1 receptors: a pivotal role in stress-induced analgesia. J Biomed Sci. 2020;27:7.
Xie X, Wisor JP, Hara J, Crowder TL, LeWinter R, Khroyan TV, et al. Hypocretin/orexin and nociceptin/orphanin FQ coordinately regulate analgesia in a mouse model of stress-induced analgesia. J Clin Invest. 2008;118:2471–81.
Dong Y, Li Y, Xiang X, Xiao Z-C, Hu J, Li Y, et al. Stress relief as a natural resilience mechanism against depression-like behaviors. Neuron. 2023;111:3789–801.e6.
Xia S-H, Hu S-W, Ge D-G, Liu D, Wang D, Zhang S, et al. Chronic pain impairs memory formation via disruption of neurogenesis mediated by mesohippocampal brain-derived neurotrophic factor signaling. Biol Psychiatry. 2020;88:597–610.
Chen S, Xu H, Dong S, Xiao L. Morpho-electric properties and diversity of oxytocin neurons in paraventricular nucleus of hypothalamus in female and male mice. J Neurosci. 2022;42:2885–904.
Lefevre A, Benusiglio D, Tang Y, Krabichler Q, Charlet A, Grinevich V. Oxytocinergic feedback circuitries: an anatomical basis for neuromodulation of social behaviors. Front Neural Circuits. 2021;15:688234.
Walker LC, Cornish LC, Lawrence AJ, Campbell EJ. The effect of acute or repeated stress on the corticotropin releasing factor system in the CRH-IRES-Cre mouse: a validation study. Neuropharmacology. 2019;154:96–106.
Jiang Z, Rajamanickam S, Justice NJ. CRF signaling between neurons in the paraventricular nucleus of the hypothalamus (PVN) coordinates stress responses. Neurobiol Stress. 2019;11:100192.
Ramot A, Jiang Z, Tian J-B, Nahum T, Kuperman Y, Justice N, et al. Hypothalamic CRFR1 is essential for HPA axis regulation following chronic stress. Nat Neurosci. 2017;20:385–8.
Neufeld-Cohen A, Kelly PAT, Paul ED, Carter RN, Skinner E, Olverman HJ, et al. Chronic activation of corticotropin-releasing factor type 2 receptors reveals a key role for 5-HT1A receptor responsiveness in mediating behavioral and serotonergic responses to stressful challenge. Biol Psychiatry. 2012;72:437–47.
Cayupe B, Troncoso B, Morgan C, Sáez-Briones P, Sotomayor-Zárate R, Constandil L, et al. The role of the paraventricular-coerulear network on the programming of hypertension by prenatal undernutrition. Int J Mol Sci. 2022;23:11965.
Dunn AJ, Swiergiel AH, Palamarchouk V. Brain circuits involved in corticotropin-releasing factor-norepinephrine interactions during stress. Ann N Y Acad Sci. 2004;1018:25–34.
Flak JN, Myers B, Solomon MB, McKlveen JM, Krause EG, Herman JP. Role of paraventricular nucleus-projecting norepinephrine/epinephrine neurons in acute and chronic stress. Eur J Neurosci. 2014;39:1903–11.
Kwon MS, Seo YJ, Shim EJ, Choi SS, Lee JY, Suh HW. The effect of single or repeated restraint stress on several signal molecules in paraventricular nucleus, arcuate nucleus and locus coeruleus. Neuroscience. 2006;142:1281–92.
Parikh D, Hamid A, Friedman TC, Nguyen K, Tseng A, Marquez P, et al. Stress-induced analgesia and endogenous opioid peptides: the importance of stress duration. Eur J Pharmacol. 2011;650:563–7.
Vaughan CW. Stressed-out endogenous cannabinoids relieve pain. Trends Pharmacol Sci. 2006;27:69–71.
Colmers PLW, Bains JS. Presynaptic mGluRs control the duration of endocannabinoid-mediated DSI. J Neurosci. 2018;38:10444–53.
Zhang Q-J, Yang J, Wang Y-M, Cong R, Zhu G-Q, Wang Z-J, et al. Effects of mu opioid receptors in paraventricular nucleus on ejaculation through mediating sympathetic nerve system activity. Neuropharmacology. 2019;158:107709.
Ji N-N, Cao S, Song X-L, Pei B, Jin C-Y, Fan B-F, et al. Glutamatergic neurons in the paraventricular nucleus of the hypothalamus participate in the regulation of visceral pain induced by pancreatic cancer in mice. Hepatobiliary Surg Nutr. 2024;13:258–72.
Ji R-R, Xu Z-Z, Gao Y-J. Emerging targets in neuroinflammation-driven chronic pain. Nat Rev Drug Discov. 2014;13:533–48.
White AG, Elias E, Orozco A, Robinson SA, Manners MT. Chronic stress-induced neuroinflammation: relevance of rodent models to human disease. Int J Mol Sci. 2024;25:5085.
Woodburn SC, Bollinger JL, Wohleb ES. The semantics of microglia activation: neuroinflammation, homeostasis, and stress. J Neuroinflammation. 2021;18:258.
Walker DM, Zhou X, Cunningham AM, Lipschultz AP, Ramakrishnan A, Cates HM, et al. Sex-specific transcriptional changes in response to adolescent social stress in the Brain’s reward circuitry. Biol Psychiatry. 2022;91:118–28.
Mogil JS. Qualitative sex differences in pain processing: emerging evidence of a biased literature. Nat Rev Neurosci. 2020;21:353–65.
Acknowledgements
We would like to acknowledge Prof. Hongxing Zhang (Xuzhou Medical University, Xuzhou, China) for the guidance in use of transgenic mice, and Drs. Chunhua Zhang, Ruyi Zhang, Huiwen Zhang, Yaxi Zhang, Ying Liang and Ran Hu (Core Facility of The First Affiliated Hospital of Nanjing Medical University, Nanjing, China) for technical support.
Funding
This study was supported by grants from the National Natural Science Foundation of China (81974171 and 82271254 to C.Y., 81720108013, 82293641 and 82130033 to J.L.C., 82401453 to S.H., 82191279 to C.H., 82301444 to Q.Z., 82201420 to D.W., 82401469 to X.Z.), the Sci-Tech Innovation 2030-Major Project (2021ZD0203100 to J.L.C.), Innovative and Entrepreneurial Team of Jiangsu Province (JSSCTD202144 to C.Y.), Jiangsu Basic Research Programs (BK20243035 to J.L.C.), Natural Science Foundation of Jiangsu Province (BK20240054 to C.Y., BK20230741 to S.H., BK20210975 to C.H.), Excellent postdoctoral program of Jiangsu Province (2023ZB599 to S.H.), China Postdoctoral Science Foundation (2023M731409 to S.H., 2023M741467 to Q.Z.) and Wu Jieping Medical Foundation (310.6750.2024-15-82 to S.H., 320.6750.2024-15-81 to Q.Z.).
Author information
Authors and Affiliations
Contributions
SH, QZ, JJY, CL, CH, JLC, and CY initiated and designed the research. SH, JH, CH and CY wrote the manuscript. SH, JH, QZ, CH, SY, ZW, YW, XZ, DW, YJ, HW, and CZ conducted experiments and analyzed the data. All the authors approved the submission.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
All experimental manipulations on mice were approved by the Institutional Animal Care and Use Committee of Nanjing Medical University (Registration number: No. IACUC-2307021) and were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. No human studies were performed. All authors read and approved the final manuscript.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hu, S., Huang, J., Zhang, Q. et al. Acute and chronic stress differentially regulate pain via distinct ensembles in the paraventricular nucleus of hypothalamus. Mol Psychiatry 31, 649–663 (2026). https://doi.org/10.1038/s41380-025-03144-4
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41380-025-03144-4