Abstract
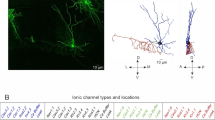
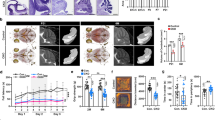
BCL11A encodes a transcription factor essential for brain development, with pathogenic variants causing intellectual disability, autism spectrum disorder (ASD), microcephaly, hypotonia, and behavioral abnormalities. While clinical studies have identified cerebellar pathology in patients with BCL11A variants, the specific roles of this gene in cerebellar function and its relationship to clinical symptoms remain unclear. In this study, we demonstrate that Bcl11a is predominantly expressed in Purkinje cells (PCs) of both the developing and adult mouse cerebellum. Conditional deletion of Bcl11a in PCs leads to impaired PC survival, disrupts dendritic morphology, reduces spine density, and results in ataxia, motor learning deficits, and autistic-like behaviors. Electrophysiological analyses reveal that Bcl11a-deficient PCs exhibit decreased frequency and regularity of spontaneous firing and reduced excitatory synaptic inputs from both parallel and climbing fibers, while maintaining normal intrinsic excitability and inhibitory synaptic inputs. Moreover, we identify Vav3 (guanosine nucleotide exchange factor 3) as a downstream target of Bcl11a in PCs and demonstrate that Vav3 overexpression partially rescues both PC dysfunction and abnormal motor and social behaviors in Bcl11a-deficient mice. Together, these findings establish Bcl11a’s critical role in PC function and provide mechanistic insight into how BCL11A mutations contribute to cerebellar dysfunction in psychiatric disorders such as ASD.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The raw sequence data reported in this paper have been deposited in the Genome Sequence Archive in National Genomics Data Center, China National Center for Bioinformation / Beijing Institute of Genomics, Chinese Academy of Sciences (GSA: CRA026084) that are publicly accessible at https://ngdc.cncb.ac.cn/gsa. The published article includes all datasets generated or analyzed during this study.
References
Kheradmand A, Zee DS. Cerebellum and ocular motor control. Front Neurol. 2011;2:53.
Thach W. A role for the cerebellum in learning movement coordination. Neurobiol Learn Mem. 1998;70:177–88.
Ioffe M. Cerebellar control of posture. Handbook of the Cerebellum and Cerebellar Disorders. Springer; 2021. p. 1379–98.
Morton SM, Bastian AJ. Cerebellar control of balance and locomotion. Neuroscientist. 2004;10:247–59.
Van Overwalle F, Manto M, Cattaneo Z, Clausi S, Ferrari C, Gabrieli JD, et al. Consensus paper: cerebellum and social cognition. Cerebellum. 2020;19:833–68.
Van Overwalle F, Manto M, Leggio M, Delgado-García JM. The sequencing process generated by the cerebellum crucially contributes to social interactions. Med Hypotheses. 2019;128:33–42.
Murdoch BE. The cerebellum and language: historical perspective and review. Cortex. 2010;46:858–68.
Mariën P, Borgatti R. Language and the cerebellum. Handb Clin Neurol. 2018;154:181–202.
Schmahmann JD. The cerebellum and cognition. Neurosci Lett. 2019;688:62–75.
Schmahmann JD, Caplan D. Cognition, emotion and the cerebellum. Brain. 2006;129:290–2.
Andreasen NC, Pierson R. The role of the cerebellum in schizophrenia. Biol Psychiatry. 2008;64:81–88.
Picard H, Amado I, Mouchet-Mages S, Olié J-P, Krebs M-O. The role of the cerebellum in schizophrenia: an update of clinical, cognitive, and functional evidences. Schizophrenia Bull. 2008;34:155–72.
Becker EB, Stoodley CJ. Autism spectrum disorder and the cerebellum. Int Rev Neurobiol. 2013;113:1–34.
Hampson DR, Blatt GJ. Autism spectrum disorders and neuropathology of the cerebellum. Front Neurosci. 2015;9:420.
D’Mello AM, Stoodley CJ. Cerebro-cerebellar circuits in autism spectrum disorder. Front Neurosci. 2015;9:408.
van der Heijden ME, Gill JS, Sillitoe RV. Abnormal cerebellar development in autism spectrum disorders. Dev Neurosci. 2021;43:181–90.
Schmahmann JD, Sherman JC. The cerebellar cognitive affective syndrome. Brain. 1998;121:561–79.
Schmahmann JD. Disorders of the cerebellum: ataxia, dysmetria of thought, and the cerebellar cognitive affective syndrome. J Neuropsychiatry Clin Neurosci. 2004;16:367–78.
Mosconi MW, Wang Z, Schmitt LM, Tsai P, Sweeney JA. The role of cerebellar circuitry alterations in the pathophysiology of autism spectrum disorders. Front Neurosci. 2015;9:156522.
Whitney ER, Kemper TL, Bauman ML, Rosene DL, Blatt GJ. Cerebellar Purkinje cells are reduced in a subpopulation of autistic brains: a stereological experiment using calbindin-D28k. Cerebellum. 2008;7:406–16.
Skefos J, Cummings C, Enzer K, Holiday J, Weed K, Levy E, et al. Regional alterations in purkinje cell density in patients with autism. PLoS ONE. 2014;9:e81255.
Peter S, Ten Brinke MM, Stedehouder J, Reinelt CM, Wu B, Zhou H, et al. Dysfunctional cerebellar Purkinje cells contribute to autism-like behaviour in Shank2-deficient mice. Nat Commun. 2016;7:12627.
Gibson JM, Vazquez AH, Yamashiro K, Jakkamsetti V, Ren C, Lei K, et al. Cerebellar contribution to autism-relevant behaviors in fragile X syndrome models. Cell Rep. 2023;42:113533.
Xu F-X, Wang X-T, Cai X-Y, Liu J-Y, Guo J-W, Yang F, et al. Purkinje-cell-specific MeCP2 deficiency leads to motor deficits and autistic-like behavior due to aberrations in PTP1B-TrkB-SK signaling. Cell Rep. 2023;42:113559.
Tsai PT, Hull C, Chu Y, Greene-Colozzi E, Sadowski AR, Leech JM, et al. Autistic-like behaviour and cerebellar dysfunction in Purkinje cell Tsc1 mutant mice. Nature. 2012;488:647–51.
Cupolillo D, Hoxha E, Faralli A, De Luca A, Rossi F, Tempia F, et al. Autistic-like traits and cerebellar dysfunction in Purkinje cell PTEN knock-out mice. Neuropsychopharmacology. 2016;41:1457–66.
Satterwhite E, Sonoki T, Willis TG, Harder L, Nowak R, Arriola EL, et al. The BCL11 gene family: involvement of BCL11A in lymphoid malignancies. Blood. 2001;98:3413–20.
Simon R, Wiegreffe C, Britsch S. Bcl11 transcription factors regulate cortical development and function. Front Mol Neurosci. 2020;13:51.
Du H, Wang Z, Guo R, Yang L, Liu G, Zhang Z, et al. Transcription factors Bcl11a and Bcl11b are required for the production and differentiation of cortical projection neurons. Cereb Cortex. 2022;32:3611–32.
Wiegreffe C, Simon R, Peschkes K, Kling C, Strehle M, Cheng J, et al. Bcl11a (Ctip1) controls migration of cortical projection neurons through regulation of Sema3c. Neuron. 2015;87:311–25.
Kuo T-Y, Hong C-J, Hsueh Y-P. Bcl11A/CTIP1 regulates expression of DCC and MAP1b in control of axon branching and dendrite outgrowth. Mol Cell Neurosci. 2009;42:195–207.
Dias C, Estruch SB, Graham SA, McRae J, Sawiak SJ, Hurst JA, et al. BCL11A haploinsufficiency causes an intellectual disability syndrome and dysregulates transcription. Am J Hum Genet. 2016;99:253–74.
Thompson CL, Ng L, Menon V, Martinez S, Lee C-K, Glattfelder K, et al. A high-resolution spatiotemporal atlas of gene expression of the developing mouse brain. Neuron. 2014;83:309–23.
Leid M, Ishmael JE, Avram D, Shepherd D, Fraulob V, Dollé P. CTIP1 and CTIP2 are differentially expressed during mouse embryogenesis. Gene Expr Patterns. 2004;4:733–9.
Visel A, Thaller C, Eichele G. GenePaint. org: an atlas of gene expression patterns in the mouse embryo. Nucleic Acids Res. 2004;32:D552–D556.
Peron A, Bradbury K, Viskochil DH, Dias C BCL11A-related intellectual disability. 2019.
Satterstrom FK, Kosmicki JA, Wang J, Breen MS, De Rubeis S, An J-Y, et al. Large-scale exome sequencing study implicates both developmental and functional changes in the neurobiology of autism. Cell. 2020;180:568–584.e523.
Peron A, D’Arco F, Aldinger KA, Smith-Hicks C, Zweier C, Gradek GA, et al. BCL11A intellectual developmental disorder: defining the clinical spectrum and genotype-phenotype correlations. Eur J Hum Genet. 2025;33:312–24.
McIlwain KL, Merriweather MY, Yuva-Paylor LA, Paylor R. The use of behavioral test batteries: effects of training history. Physiol Behav. 2001;73:705–17.
Crawley JN. What’s wrong with my mouse?: behavioral phenotyping of transgenic and knockout mice. John Wiley & Sons; 2007.
Van Putten M, Aartsma-Rus A, Louvain-la-Neuve L. The use of hanging wire tests to monitor muscle strength and condition over time. TREAT-NMD Neuromscular Network/Wellstone Muscular Dystrophy Center, Washington, DC. 2011;2:1–11.
Liu D, Nanclares C, Simbriger K, Fang K, Lorsung E, Le N, et al. Autistic-like behavior and cerebellar dysfunction in Bmal1 mutant mice ameliorated by mTORC1 inhibition. Mol Psychiatry. 2023;28:3727–38.
Su L-D, Shen Y. Blockade of glutamate transporters facilitates cerebellar synaptic long-term depression. Neuroreport. 2009;20:502–7.
Guo X, Yuan Y, Su X, Cao Z, Chu C, Lei C, et al. Different projection neurons of basolateral amygdala participate in the retrieval of morphine withdrawal memory with diverse molecular pathways. Mol Psychiatry. 2024;29:793–808.
Yang G, Yang Y, Song Z, Chen L, Liu F, Li Y, et al. Spliceosomal GTPase Eftud2 deficiency-triggered ferroptosis leads to Purkinje cell degeneration. Neuron. 2024;112:3452–3469.e3459.
Vladoiu MC, El-Hamamy I, Donovan LK, Farooq H, Holgado BL, Sundaravadanam Y, et al. Childhood cerebellar tumours mirror conserved fetal transcriptional programs. Nature. 2019;572:67–73.
Kozareva V, Martin C, Osorno T, Rudolph S, Guo C, Vanderburg C, et al. A transcriptomic atlas of mouse cerebellar cortex comprehensively defines cell types. Nature. 2021;598:214–9.
Oberdick J, Smeyne RJ, Mann JR, Zackson S, Morgan JI. A promoter that drives transgene expression in cerebellar purkinje and retinal bipolar neurons. Science. 1990;248:223–6.
Barski JJ, Dethleffsen K, Meyer M. Cre recombinase expression in cerebellar Purkinje cells. Genesis. 2000;28:93–98.
Fatemi SH, Halt AR, Realmuto G, Earle J, Kist DA, Thuras P, et al. Purkinje cell size is reduced in cerebellum of patients with autism. Cell Mol Neurobiol. 2002;22:171–5.
Soblet J, Dimov I, Graf von Kalckreuth C, Cano‐Chervel J, Baijot S, Pelc K, et al. BCL11A frameshift mutation associated with dyspraxia and hypotonia affecting the fine, gross, oral, and speech motor systems. Am J Med Genet Part A. 2018;176:201–8.
Liu X-X, Chen X-H, Zheng Z-W, Jiang Q, Li C, Yang L, et al. BOD1 regulates the cerebellar IV/V lobe-fastigial nucleus circuit associated with motor coordination. Signal Transduct Target Ther. 2022;7:170.
Vinueza Veloz M, Buijsen R, Willemsen R, Cupido A, Bosman LW, Koekkoek SK, et al. The effect of an mGluR5 inhibitor on procedural memory and avoidance discrimination impairments in Fmr1 KO mice. Genes Brain Behav. 2012;11:325–31.
Vinueza Veloz MF, Zhou K, Bosman LW, Potters J-W, Negrello M, Seepers RM, et al. Cerebellar control of gait and interlimb coordination. Brain Structure Funct. 2015;220:3513–36.
Takeo YH, Shuster SA, Jiang L, Hu MC, Luginbuhl DJ, Rülicke T, et al. GluD2-and Cbln1-mediated competitive interactions shape the dendritic arbors of cerebellar Purkinje cells. Neuron. 2021;109:629–644.e628.
Silvestri L, Paciscopi M, Soda P, Biamonte F, Iannello G, Frasconi P, et al. Quantitative neuroanatomy of all Purkinje cells with light sheet microscopy and high-throughput image analysis. Front Neuroanat. 2015;9:68.
Woodruff-Pak D. Stereological estimation of Purkinje neuron number in C57BL/6 mice and its relation to associative learning. Neuroscience. 2006;141:233–43.
Bustelo XR. Vav family exchange factors: an integrated regulatory and functional view. Small GTPases. 2014;5:e973757.
Wegrzyn D, Wegrzyn C, Tedford K, Fischer K-D, Faissner A. Deletion of the nucleotide exchange factor Vav3 enhances axonal complexity and synapse formation but tampers activity of hippocampal neuronal networks in vitro. Int J Mol Sci. 2020;21:856.
Quevedo C, Sauzeau V, Menacho-Márquez M, Castro-Castro A, Bustelo XR. Vav3-deficient mice exhibit a transient delay in cerebellar development. Mol Biol Cell. 2010;21:1125–39.
Hancarova M, Simandlova M, Drabova J, Mannik K, Kurg A, Sedlacek Z. A patient with de novo 0.45 Mb deletion of 2p16. 1: the role of BCL11A, PAPOLG, REL, and FLJ16341 in the 2p15‐p16. 1 microdeletion syndrome. Am J Med Genet Part A. 2013;161:865–70.
Balci TB, Sawyer SL, Davila J, Humphreys P, Dyment DA. Brain malformations in a patient with deletion 2p16. 1: A refinement of the phenotype to BCL11A. Eur J Med Genet. 2015;58:351–4.
Lévy J, Coussement A, Dupont C, Guimiot F, Baumann C, Viot G, et al. Molecular and clinical delineation of 2p15p16. 1 microdeletion syndrome. Am J Med Genet Part A. 2017;173:2081–7.
John A, Brylka H, Wiegreffe C, Simon R, Liu P, Jüttner R, et al. Bcl11a is required for neuronal morphogenesis and sensory circuit formation in dorsal spinal cord development. Development. 2012;139:1831–41.
Tolve M, Ulusoy A, Patikas N, Islam KUS, Bodea GO, Öztürk E, et al. The transcription factor BCL11A defines distinct subsets of midbrain dopaminergic neurons. Cell Rep. 2021;36:109697.
Greig LC, Woodworth MB, Greppi C, Macklis JD. Ctip1 controls acquisition of sensory area identity and establishment of sensory input fields in the developing neocortex. Neuron. 2016;90:261–77.
Wang R, Tan J, Guo J, Zheng Y, Han Q, So K-F, et al. Aberrant development and synaptic transmission of cerebellar cortex in a VPA induced mouse autism model. Front Cell Neurosci. 2018;12:500.
Main SL, Kulesza RJ. Repeated prenatal exposure to valproic acid results in cerebellar hypoplasia and ataxia. Neuroscience. 2017;340:34–47.
Ehret G, Bernecker C. Low-frequency sound communication by mouse pups (Mus musculus): wriggling calls release maternal behaviour. Anim Behav. 1986;34:821–30.
Hanzel M, Fernando K, Maloney SE, Horn Z, Gong S, Mätlik K, et al. Mice lacking Astn2 have ASD-like behaviors and altered cerebellar circuit properties. Proc Natl Acad Sci. 2024;121:e2405901121.
Korenke GC, Schulte B, Biskup S, Neidhardt J, Owczarek-Lipska M. A novel de novo frameshift mutation in the BCL11A gene in a patient with intellectual disability syndrome and epilepsy. Mol Syndromol. 2020;11:135–40.
Beleford DT, Van Ziffle J, Hodoglugil U, Slavotinek AM. A missense variant, p.(Ile269Asn), in MC4R as a secondary finding in a child with BCL11A-related intellectual disability. Eur J Med Genet. 2020;63:103969.
Bruce L, Peter B. Three children with different de novo BCL11A variants and diverse developmental phenotypes, but shared global motor discoordination and apraxic speech: Evidence for a functional gene network influencing the developing cerebellum and motor and auditory cortices. Am J Med Genet Part A. 2022;188:3401–15.
Matson ML, Matson JL, Beighley JS. Comorbidity of physical and motor problems in children with autism. Res Dev Disabil. 2011;32:2304–8.
Licari MK, Alvares GA, Varcin K, Evans KL, Cleary D, Reid SL, et al. Prevalence of motor difficulties in autism spectrum disorder: analysis of a population-based cohort. Autism Res. 2020;13:298–306.
Koekkoek S, Yamaguchi K, Milojkovic B, Dortland B, Ruigrok T, Maex R, et al. Deletion of FMR1 in Purkinje cells enhances parallel fiber LTD, enlarges spines, and attenuates cerebellar eyelid conditioning in Fragile X syndrome. Neuron. 2005;47:339–52.
Hoebeek F, Stahl J, Van Alphen A, Schonewille M, Luo C, Rutteman M, et al. Increased noise level of purkinje cell activities minimizes impact of their modulation during sensorimotor control. Neuron. 2005;45:953–65.
Aldinger KA, Thomson Z, Phelps IG, Haldipur P, Deng M, Timms AE, et al. Spatial and cell type transcriptional landscape of human cerebellar development. Nat Neurosci. 2021;24:1163–75.
Movilla N, Bustelo XR. Biological and regulatory properties of Vav-3, a new member of the Vav family of oncoproteins. Mol Cell Biol. 1999;19:7870–85.
Aoki K, Nakamura T, Fujikawa K, Matsuda M. Local phosphatidylinositol 3, 4, 5-trisphosphate accumulation recruits Vav2 and Vav3 to activate Rac1/Cdc42 and initiate neurite outgrowth in nerve growth factor-stimulated PC12 cells. Mol Biol Cell. 2005;16:2207–17.
Toumaniantz G, Ferland-McCollough D, Cario-Toumaniantz C, Pacaud P, Loirand G. The Rho protein exchange factor Vav3 regulates vascular smooth muscle cell proliferation and migration. Cardiovascular Res. 2010;86:131–40.
Hornstein I, Alcover A, Katzav S. Vav proteins, masters of the world of cytoskeleton organization. Cell Signal. 2004;16:1–11.
Ulc A, Gottschling C, Schäfer I, Wegrzyn D, van Leeuwen S, Luft V, et al. Involvement of the guanine nucleotide exchange factor Vav3 in central nervous system development and plasticity. Biol Chem. 2017;398:663–75.
Luft V, Reinhard J, Shibuya M, Fischer KD, Faissner A. The guanine nucleotide exchange factor Vav3 regulates differentiation of progenitor cells in the developing mouse retina. Cell Tissue Res. 2015;359:423–40.
Peter S, De Zeeuw CI, Boeckers TM, Schmeisser MJ. Cerebellar and striatal pathologies in mouse models of autism spectrum disorder. Transl Anat Cell Biol Autism Spectr Disord. 2017;224:103–19.
Acknowledgements
This work was supported by the National Key Research and Development Program of China (2021ZD0202500), the Natural Science Foundation of China (31930044, 31725012), the Foundation of Shanghai Municipal Education Commission (2019-01-07-00-07-E00062), the Collaborative Innovation Program of Shanghai Municipal Health Commission (2020CXJQ01), the Shanghai Municipal Science and Technology Major Project (No.2018SHZDZX01) and ZJLab to YCY.
Author information
Authors and Affiliations
Contributions
YCY conceived the study. YCY and JZ wrote the manuscript. YCY, LYL, QH, and JZ designed the experiments. JZ performed the immunohistochemistry, behavioral tests, MRI acquisition, single-cell sequencing analysis, and virus injection experiments. YXL conducted the patch-clamp recordings. JZ and YXL analyzed the data. QH assisted with the behavioral data analysis. YY contributed to the electrophysiology and immunohistochemistry experiments. JYC assisted with standardized MRI acquisition. FWY provided support for the immunohistochemistry experiments. LY assisted with single-cell data analysis. All authors contributed to manuscript revision, read, and approved the submitted version.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The study was approved by Ethics Committee of Fudan University (approval number: FE21173). All methods were performed in accordance with the ethical standards of Fudan University and complied with the relevant institutional and national guidelines and regulations for the care and use of laboratory animals. This study did not involve human participants, and informed consent was not required. No identifiable images from human participants are included in this article.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, J., Li, YX., Huang, Q. et al. Bcl11a deficiency in cerebellar Purkinje cells causes ataxia and autistic-like behavior by altering Vav3. Mol Psychiatry 31, 802–818 (2026). https://doi.org/10.1038/s41380-025-03175-x
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41380-025-03175-x