Abstract
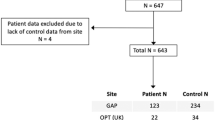
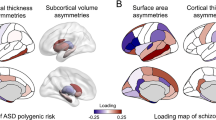
Cortical thickness asymmetry has been proposed as a latent biomarker for autism spectrum disorder (ASD) and schizophrenia (SZ). However, the degree of abnormal asymmetry at the individual level in ASD and SZ remains unclear. To investigate this, we employed a normative modeling approach. Normative ranges for the whole brain and regional (160 cortical parcels) cortical thickness asymmetry index (AI) were established using a training set of healthy subjects (n = 4904, 45.15% male, age range: 6–95 years), controlling for age, sex, image quality, and scanner. We calculated z-scores to quantify individual deviations from the normative median in a test set consisting of healthy controls (HCtest, n = 526, 40% male), participants with ASD (n = 135, 83% male), and SZ (n = 287, 81% male). Regional deviance was assessed by counting the number of individuals with significant deviations below (infra-normal, z-score ≤ −1.96) or above (supra-normal, z-score ≥ 1.96) the normative median in each parcel. We also evaluated individual deviance by counting the number of regions with significant deviations for each participant. A multivariate approach was employed to determine whether regional deviance could separate the three groups. There were no differences for deviance of whole brain AI between any of the groups. Distributions of individual deviances overlapped across all 160 regions, with one superior temporal region in which SZ individuals showed a higher proportion of supra-normal AI values compared to HCtest (HCtest = 1.14%, SZ = 5.92%, χ2 = 15.45, PFDR < 0.05, ω = 0.14). The SZ group had a higher average number of regions with significant deviations than HCtest (infra-normal: z = 4.21, p < 0.01; supra-normal: z = 4.33, p < 0.01) but this group difference had limited predictive diagnostic accuracy at the individual level (Area Under the Curve≅60%). The multivariate analysis showed no association between regional deviance and diagnosis. Results were consistent when using a different parcellation, alternative asymmetry calculations, analysis restricted to males, and after controlling for handedness and IQ. Normative modelling revealed little to no evidence of atypical individualized cortical thickness asymmetry in ASD and SZ. The results of this study challenge the utility of cortical thickness asymmetry as a biomarker for ASD and SZ.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
In addition to non-publicly available datasets, the following publicly available datasets were used: Aomic (id1000, piop1 and piop2) available at https://openneuro.org/datasets/ds003097, https://openneuro.org/datasets/ds002785 and https://openneuro.org/datasets/ds002790; camcan available at https://camcan-archive.mrc-cbu.cam.ac.uk; dlbs available at https://fcon_1000.projects.nitrc.org/indi/retro/dlbs.html; ixi available at http://brain-development.org/ixidataset; narratives available at https://openneuro.org/datasets/ds002345; oasis3 available at www.oasis-brains.org; rockland available at https://rocklandsample.org/; sald available at https://fcon_1000.projects.nitrc.org/indi/retro/sald.html; ABIDE-I and II available at https://fcon_1000.projects.nitrc.org/indi/abide/; MITASD available at https://openneuro.org/datasets/ds000212/versions/1.0.0; WASHASD available at https://openneuro.org/datasets/ds002522/versions/1.0.0; BGS available at http://schizconnect.org/; COBRE available at http://schizconnect.org/.
Code availability
All code used to perform the analyses can be found at https://github.com/iamjoostjanssen/NormModel_AI_SZ_ASD.
References
Zhou D, Lebel C, Evans A, Beaulieu C. Cortical thickness asymmetry from childhood to older adulthood. Neuroimage. 2013;83:66–74.
Toga AW, Thompson PM. Mapping brain asymmetry. Nat Rev Neurosci. 2003;4:37–48.
Corballis MC. Left brain, right brain: facts and fantasies. PLoS Biol. 2014;12:e1001767.
Postema MC, van Rooij D, Anagnostou E, Arango C, Auzias G, Behrmann M, et al. Altered structural brain asymmetry in autism spectrum disorder in a study of 54 datasets. Nat Commun. 2019;10:4958.
Sha Z, Schijven D, Francks C. Patterns of brain asymmetry associated with polygenic risks for autism and schizophrenia implicate language and executive functions but not brain masculinization. Mol Psychiatry. 2021;26:7652–60.
Floris DL, Lai MC, Auer T, Lombardo MV, Ecker C, Chakrabarti B, et al. Atypically rightward cerebral asymmetry in male adults with autism stratifies individuals with and without language delay. Hum Brain Mapp. 2016;37:230–53.
Li Q, Zhao W, Palaniyappan L, Guo S. Atypical hemispheric lateralization of brain function and structure in autism: a comprehensive meta-analysis study. Psychol Med. 2023;53:1–12.
Lindell AK, Hudry K. Atypicalities in cortical structure, handedness, and functional lateralization for language in autism spectrum disorders. Neuropsychol Rev. 2013;23:257–70.
Brugger SP, Howes OD. Heterogeneity and homogeneity of regional brain structure in schizophrenia: a meta-analysis. JAMA Psychiatry. 2017;74:1104–11.
Kong XZ, Postema MC, Guadalupe T, de Kovel C, Boedhoe PSW, Hoogman M, et al. Mapping brain asymmetry in health and disease through the ENIGMA consortium. Hum Brain Mapp. 2022 Jan;43:167–81.
Schijven D, Postema MC, Fukunaga M, Matsumoto J, Miura K, de Zwarte SMC, et al. Large-scale analysis of structural brain asymmetries in schizophrenia via the ENIGMA consortium. Proc Natl Acad Sci USA. 2023;120:e2213880120.
Crow TJ. Schizophrenia as failure of hemispheric dominance for language. Trends Neurosci. 1997;20:339–43.
Marquand AF, Kia SM, Zabihi M, Wolfers T, Buitelaar JK, Beckmann CF. Conceptualizing mental disorders as deviations from normative functioning. Mol Psychiatry. 2019;24:1415–24.
Bethlehem RaI, Seidlitz J, White SR, Vogel JW, Anderson KM, Adamson C, et al. Brain charts for the human lifespan. Nature. 2022;604:525–33.
Segal A, Parkes L, Aquino K, Kia SM, Wolfers T, Franke B, et al. Regional, circuit and network heterogeneity of brain abnormalities in psychiatric disorders. Nat Neurosci. 2023;26:1613–29.
Bedford SA, Ortiz-Rosa A, Schabdach JM, Costantino M, Tullo S, Piercy T, et al. The impact of quality control on cortical morphometry comparisons in autism. Imaging Neurosci Camb Mass. 2023;1:1–21.
Chopra S, Segal A, Oldham S, Holmes A, Sabaroedin K, Orchard ER, et al. Network-based spreading of gray matter changes across different stages of psychosis. JAMA Psychiatry. 2023;80:1246–57.
Janssen J, Díaz-Caneja CM, Alloza C, Schippers A, de Hoyos L, Santonja J, et al. Dissimilarity in sulcal width patterns in the cortex can be used to identify patients with schizophrenia with extreme deficits in cognitive performance. Schizophr Bull. 2021;47:552–61.
Gaser C, Dahnke R, Thompson PM, Kurth F, Luders E. The Alzheimer’s Disease Neuroimaging Initiative null. CAT: a computational anatomy toolbox for the analysis of structural MRI data. Gigascience. 2024;13:giae049.
Wolfers T, Doan NT, Kaufmann T, Alnæs D, Moberget T, Agartz I, et al. Mapping the heterogeneous phenotype of schizophrenia and bipolar disorder using normative models. JAMA Psychiatry. 2018;75:1146–55.
Fischl B. FreeSurfer. Neuroimage. 2012;62:774–81.
Rosen AFG, Roalf DR, Ruparel K, Blake J, Seelaus K, Villa LP, et al. Quantitative assessment of structural image quality. Neuroimage. 2018;169:407–18.
Rutherford S, Barkema P, Tso IF, Sripada C, Beckmann CF, Ruhe HG, et al. Evidence for embracing normative modeling. eLife. 2023;12:e85082.
Romero-Garcia R, Atienza M, Cantero JL. Predictors of coupling between structural and functional cortical networks in normal aging. Hum Brain Mapp. 2014;35:2724–40.
Roe JM, Vidal-Pineiro D, Amlien IK, Pan M, Sneve MH, Thiebaut de Schotten M, et al. Tracing the development and lifespan change of population-level structural asymmetry in the cerebral cortex. eLife. 2023;12:e84685.
Greve DN, Van der Haegen L, Cai Q, Stufflebeam S, Sabuncu MR, Fischl B, et al. A surface-based analysis of language lateralization and cortical asymmetry. J Cogn Neurosci. 2013;25:1477–92.
Bahar N, Cler GJ, Krishnan S, Asaridou SS, Smith HJ, Willis HE, et al. Differences in cortical surface area in developmental language disorder. Neurobiol Lang Camb Mass. 2024;5:288–314.
Schaefer A, Kong R, Gordon EM, Laumann TO, Zuo XN, Holmes AJ, et al. Local-global parcellation of the human cerebral cortex from intrinsic functional connectivity MRI. Cereb Cortex N Y N 1991. 2018;28:3095–114.
Kruggel F, Solodkin A. Heritability of structural patterning in the human cerebral cortex. Neuroimage. 2020;221:117169.
Kuhn M. Building predictive models in R using the caret package. J Stat Softw. 2008;28:1–26.
Fraza CJ, Dinga R, Beckmann CF, Marquand AF. Warped Bayesian linear regression for normative modelling of big data. Neuroimage. 2021;245:118715.
Bethlehem RAI, Seidlitz J, Romero-Garcia R, Trakoshis S, Dumas G, Lombardo MV. A normative modelling approach reveals age-atypical cortical thickness in a subgroup of males with autism spectrum disorder. Commun Biol. 2020;3:486.
Lv J, Di Biase M, Cash RFH, Cocchi L, Cropley VL, Klauser P, et al. Individual deviations from normative models of brain structure in a large cross-sectional schizophrenia cohort. Mol Psychiatry. 2021;26:3512–23.
ENIGMA Clinical High Risk for Psychosis Working Group, Haas SS, Ge R, Agartz I, Amminger GP, Andreassen OA, et al. Normative modeling of brain morphometry in clinical high risk for psychosis. JAMA Psychiatry. 2024;81:77–88.
Huang AS, Kang K, Vandekar S, Rogers BP, Heckers S, Woodward ND Lifespan development of thalamic nuclei and characterizing thalamic nuclei abnormalities in schizophrenia using normative modeling. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. 2024;49:1518–27.
Hothorn T, Hornik K, van de Wiel MA, Zeileis A. Implementing a class of permutation tests: the coin package. J Stat Softw. 2008;28:1–23.
Van Der Maaten L. Accelerating t-SNE using tree-based algorithms. J Mach Learn Res. 2014;15:3221–45.
Hennig C, Liao TF. How to find an appropriate clustering for mixed-type variables with application to socio-economic stratification. J R Stat Soc Ser C Appl Stat. 2013;62:309–69.
Yarkoni T, Poldrack RA, Nichols TE, Van Essen DC, Wager TD. Large-scale automated synthesis of human functional neuroimaging data. Nat Methods. 2011;8:665–70.
Robin X, Turck N, Hainard A, Tiberti N, Lisacek F, Sanchez JC, et al. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics. 2011;12:77.
van Erp TGM, Walton E, Hibar DP, Schmaal L, Jiang W, Glahn DC, et al. Cortical brain abnormalities in 4474 Individuals with schizophrenia and 5098 control subjects via the enhancing neuro imaging genetics through meta analysis (ENIGMA) consortium. Biol Psychiatry. 2018;84:644–54.
Zabihi M, Oldehinkel M, Wolfers T, Frouin V, Goyard D, Loth E, et al. Dissecting the heterogeneous cortical anatomy of autism spectrum disorder using normative models. Biol Psychiatry Cogn Neurosci Neuroimaging. 2019;4:567–78.
Floris DL, Wolfers T, Zabihi M, Holz NE, Zwiers MP, Charman T, et al. Atypical brain asymmetry in Autism-A candidate for clinically meaningful stratification. Biol Psychiatry Cogn Neurosci Neuroimaging. 2021;6:802–12.
Cullen AE, De Brito SA, Gregory SL, Murray RM, Williams SCR, Hodgins S, et al. Temporal lobe volume abnormalities precede the prodrome: a study of children presenting antecedents of schizophrenia. Schizophr Bull. 2013;39:1318–27.
Crow TJ. Temporal lobe asymmetries as the key to the etiology of schizophrenia. Schizophr Bull. 1990;16:433–43.
Winter NR, Blanke J, Leenings R, Ernsting J, Fisch L, Sarink K, et al. A systematic evaluation of machine learning-based biomarkers for major depressive disorder. JAMA Psychiatry. 2024;81:386–95.
Porter A, Fei S, Damme KSF, Nusslock R, Gratton C, Mittal VA. A meta-analysis and systematic review of single vs. multimodal neuroimaging techniques in the classification of psychosis. Mol Psychiatry. 2023;28:3278–92.
Palaniyappan L, Liddle PF. Does the salience network play a cardinal role in psychosis? An emerging hypothesis of insular dysfunction. J Psychiatry Neurosci JPN. 2012;37:17–27.
Wheeler AL, Voineskos AN. A review of structural neuroimaging in schizophrenia: from connectivity to connectomics. Front Hum Neurosci. 2014;8:653.
Hong SJ, Valk SL, Di Martino A, Milham MP, Bernhardt BC. Multidimensional neuroanatomical subtyping of autism spectrum disorder. Cereb Cortex N Y N 1991. 2018;28:3578–88.
Chen YC, Arnatkevičiūtė A, McTavish E, Pang JC, Chopra S, Suo C, et al. The individuality of shape asymmetries of the human cerebral cortex. eLife. 2022;11:e75056.
Chen YC, Tiego J, Segal A, Chopra S, Holmes A, Suo C, et al. A multiscale characterization of cortical shape asymmetries in early psychosis. Brain Commun. 2024;6:fcae015.
Acknowledgements
Supported by the Spanish Ministry of Science and Innovation, Instituto de Salud Carlos III (ISCIII), CIBER -Consorcio Centro de Investigación Biomédica en Red- (CB/07/09/0023), co-financed by the European Union, ERDF Funds from the European Commission, “A way of making Europe”, (PI16/02012, PI17/01249, PI17/00997, PI19/01024, PI20/00721, PI22/01824, PI22/01621, PI23/00625), financed by the European Union - NextGenerationEU (PMP21/00051), Madrid Regional Government (S2022/BMD-7216 AGES 3-CM), European Union Seventh Framework Program, European Union H2020 Program under the Innovative Medicines Initiative 2 Joint Undertaking: Project PRISM-2 (Grant agreement No.101034377), Project COllaborative Network for European Clinical Trials For Children “c4c” (Grant agreement No 777389) Horizon Europe, the National Institute of Mental Health of the National Institutes of Health under Award Number 1U01MH124639-01 (Project ProNET), Award Number 5P50MH115846-03 (Project FEP-CAUSAL) and Award Number 1R01MH128971-01A1 (Project SZ-aging), Fundación Familia Alonso, and Fundación Alicia Koplowitz. The results leading to this publication have received funding from the Innovative Medicines Initiative 2 Joint Undertaking under grant agreement No 777394 for the project AIMS-2-TRIALS. This Joint Undertaking receives support from the European Union’s Horizon 2020 research and innovation programme and EFPIA and AUTISM SPEAKS, Autistica, SFARI. Any views expressed are those of the author(s) and not necessarily those of the funders (IHI-JU2). The authors thank Yasser Alemán-Goméz, Alberto Fernández Pena, Zimbo Boudewijns, and Joyce van Baaren for code and technical assistance.
Author information
Authors and Affiliations
Contributions
Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work: Joost Janssen, Marta Martín Echave, Hugo G. Schnack, Covadonga M. Díaz-Caneja. Drafting the work or revising it critically for important intellectual content: Joost Janssen, Marta Martín Echave, Hugo G. Schnack, Pedro M. Gordaliza, Neeltje E.M. van Haren, Celso Arango. Final approval of the version to be published: Joost Janssen, Marta Martín Echave, Covadonga M. Díaz-Caneja, Niels Janssen, Pedro M. Gordaliza, Elizabeth E.L. Buimer, Neeltje E.M. van Haren, Wiepke Cahn, Celso Arango, René S. Kahn, Hilleke E. Hulshoff Pol, Hugo G. Schnack. Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved: Joost Janssen, Marta Martín Echave, Hugo G. Schnack.
Corresponding author
Ethics declarations
Competing interests
No authors declare any competing financial interests in relation to the work described. Dr. Díaz-Caneja has received honoraria from Angelini and Viatris. Dr. Arango has been a consultant to or has received honoraria or grants from Acadia, Angelini, Gedeon Richter, Janssen-Cilag, Lundbeck, Otsuka, Roche, Sage, Servier, Shire, Schering-Plough, Sumitomo Dainippon Pharma, Sunovion, and Takeda. Dr. Cahn has received unrestricted research grants from or served as an independent symposium speaker or consultant for Eli Lilly, Bristol-Myers Squibb, Lundbeck, Sanofi-Aventis, Janssen-Cilag, AstraZeneca, and Schering-Plough. The other authors report no financial relationships with commercial interests.
Ethics approval and consent to participate
All methods were performed in accordance with the relevant guidelines and regulations. All sites obtained local institutional review board approval. Written informed consent was obtained from every participant, or from the participant’s guardian for minors. All studies were conducted in accordance with the Declaration of Helsinki.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Martín Echave, M., Schnack, H.G., Díaz-Caneja, C.M. et al. Individualized cortical thickness asymmetry in autism spectrum disorder and schizophrenia. Mol Psychiatry (2025). https://doi.org/10.1038/s41380-025-03359-5
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41380-025-03359-5