Abstract
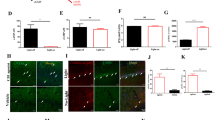
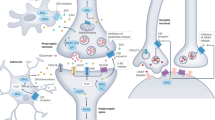
Electroencephalographic (EEG) recordings in individuals with Fragile X Syndrome (FXS) and the mouse model of FXS (Fmr1 KO) display cortical hyperexcitability at rest, as well as deficits in sensory-driven cortical network synchrony. A form of circuit hyperexcitability is observed in ex vivo cortical slices of Fmr1 KO mice as prolonged persistent activity, or Up, states. It is unknown if the circuit mechanisms that cause prolonged Up states contribute to FXS-relevant EEG phenotypes. Here we examined the role of endocannabinoids (eCB) in prolonged Up states in slices and resting and sensory-driven EEG phenotypes in awake Fmr1 KO mice. Bidirectional changes in eCB function are reported in the Fmr1 KO that depend on synapse type (excitatory or inhibitory). We demonstrate that pharmacological or genetic reduction of Cannabinoid Receptor 1 (CB1R) in GABAergic neurons rescues prolonged cortical Up states and deficits in sensory-driven cortical synchrony in Fmr1 KO mice. In support of these findings, recordings from Fmr1 KO cortical Layer (L) 2/3 pyramidal neurons revealed enhanced CB1R-mediated suppression of inhibitory synaptic currents. In contrast, genetic reduction of Cnr1 in glutamatergic neurons did not affect Up state duration, but deletion of Fmr1 in the same neurons was sufficient to cause long Up states. These findings support a model where loss of Fmr1 in glutamatergic neurons leads to enhanced CB1R-mediated suppression of GABAergic synaptic transmission, prolonged cortical circuit activation and reduced sensory-driven circuit synchronization. Results suggest that antagonism of CB1Rs may be a therapeutic strategy to correct sensory processing deficits in FXS.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The authors declare that all data supporting the findings of this study are available in the main text or the supplementary materials. Datasets are available from the corresponding authors upon request. Correspondence and requests for materials should be addressed to KMH and DKB (Kimberly.Huber@UTSouthwestern.edu and devin.binder@ucr.edu). Labview software for analysis of Up states is available at https://github.com/jaygibson244/GibsonHuber-UP-states.git. The MATLAB code used to perform statistical analysis for chirp, 40 and 80 Hz ASSR is available at Github: https://github.com/CarrieRJonak/MEA-EEG_ITPC.
References
Bassell GJ, Warren ST. Fragile X syndrome: loss of local mRNA regulation alters synaptic development and function. Neuron. 2008;60:201–14.
Juczewski K, von Richthofen H, Bagni C, Celikel T, Fisone G, Krieger P. Somatosensory map expansion and altered processing of tactile inputs in a mouse model of fragile X syndrome. Neurobiol Dis. 2016;96:201–15.
He CX, Cantu DA, Mantri SS, Zeiger WA, Goel A, Portera-Cailliau C. Tactile defensiveness and impaired adaptation of neuronal activity in the Fmr1 knock-out mouse model of autism. J Neurosci. 2017;37:6475–87.
McCullagh EA, Rotschafer SE, Auerbach BD, Klug A, Kaczmarek LK, Cramer KS, et al. Mechanisms underlying auditory processing deficits in Fragile X syndrome. FASEB J. 2020;34:3501–18.
Wang J, Ethridge LE, Mosconi MW, White SP, Binder DK, Pedapati EV, et al. A resting EEG study of neocortical hyperexcitability and altered functional connectivity in fragile X syndrome. J Neurodev Disord. 2017;9:11.
Lovelace JW, Ethell IM, Binder DK, Razak KA. Translation-relevant EEG phenotypes in a mouse model of fragile X syndrome. Neurobiol Dis. 2018;115:39–48.
Ethridge LE, White SP, Mosconi MW, Wang J, Pedapati EV, Erickson CA, et al. Neural synchronization deficits linked to cortical hyper-excitability and auditory hypersensitivity in fragile X syndrome. Mol Autism. 2017;8:22.
Jonak CR, Lovelace JW, Ethell IM, Razak KA, Binder DK. Multielectrode array analysis of EEG biomarkers in a mouse model of Fragile X Syndrome. Neurobiol Dis. 2020;138:104794.
Jonak CR, Assad SA, Garcia TA, Sandhu MS, Rumschlag JA, Razak KA, et al. Phenotypic analysis of multielectrode array EEG biomarkers in developing and adult male Fmr1 KO mice. Neurobiol Dis. 2024;195:106496.
Ethridge LE, De Stefano LA, Schmitt LM, Woodruff NE, Brown KL, Tran M, et al. Auditory EEG biomarkers in fragile X syndrome: clinical relevance. Front Integr Neurosci. 2019;13:60.
Pedapati EV, Schmitt LM, Ethridge LE, Miyakoshi M, Sweeney JA, Liu R, et al. Neocortical localization and thalamocortical modulation of neuronal hyperexcitability contribute to fragile X syndrome. Commun Biol. 2022;5:442.
Schmitt LM, Li J, Liu R, Horn PS, Sweeney JA, Erickson CA, et al. Altered frontal connectivity as a mechanism for executive function deficits in fragile X syndrome. Mol Autism. 2022;13:47.
Gibson JR, Bartley AF, Hays SA, Huber KM. Imbalance of neocortical excitation and inhibition and altered UP states reflect network hyperexcitability in the mouse model of fragile X syndrome. J Neurophysiol. 2008;100:2615–26.
Hays SA, Huber KM, Gibson JR. Altered neocortical rhythmic activity states in Fmr1 KO mice are due to enhanced mGluR5 signaling and involve changes in excitatory circuitry. J Neurosci. 2011;31:14223–34.
Osterweil EK, Chuang SC, Chubykin AA, Sidorov M, Bianchi R, Wong RK, et al. Lovastatin corrects excess protein synthesis and prevents epileptogenesis in a mouse model of fragile X syndrome. Neuron. 2013;77:243–50.
Zhang Y, Bonnan A, Bony G, Ferezou I, Pietropaolo S, Ginger M, et al. Dendritic channelopathies contribute to neocortical and sensory hyperexcitability in Fmr1(-/y) mice. Nat Neurosci. 2014;17:1701–9.
Contractor A, Klyachko VA, Portera-Cailliau C. Altered neuronal and circuit excitability in fragile X syndrome. Neuron. 2015;87:699–715.
Liu X, Kumar V, Tsai NP, Auerbach BD. Hyperexcitability and homeostasis in fragile X syndrome. Front Mol Neurosci. 2021;14:805929.
Cea-Del Rio CA, Nunez-Parra A, Freedman SM, Kushner JK, Alexander AL, Restrepo D, et al. Disrupted inhibitory plasticity and homeostasis in fragile X syndrome. Neurobiol Dis. 2020;142:104959.
Rotschafer S, Razak K. Altered auditory processing in a mouse model of fragile X syndrome. Brain Res. 2013;1506:12–24.
Jung KM, Sepers M, Henstridge CM, Lassalle O, Neuhofer D, Martin H, et al. Uncoupling of the endocannabinoid signalling complex in a mouse model of fragile X syndrome. Nat Commun. 2012;3:1080.
Zhang L, Alger BE. Enhanced endocannabinoid signaling elevates neuronal excitability in fragile X syndrome. J Neurosci. 2010;30:5724–9.
Maccarrone M, Rossi S, Bari M, De Chiara V, Rapino C, Musella A, et al. Abnormal mGlu 5 receptor/endocannabinoid coupling in mice lacking FMRP and BC1 RNA. Neuropsychopharmacology. 2010;35:1500–9.
Tang AH, Alger BE. Homer protein-metabotropic glutamate receptor binding regulates endocannabinoid signaling and affects hyperexcitability in a mouse model of fragile X syndrome. J Neurosci. 2015;35:3938–45.
Busquets-Garcia A, Gomis-Gonzalez M, Guegan T, Agustin-Pavon C, Pastor A, Mato S, et al. Targeting the endocannabinoid system in the treatment of fragile X syndrome. Nat Med. 2013;19:603–7.
Wilson RI, Kunos G, Nicoll RA. Presynaptic specificity of endocannabinoid signaling in the hippocampus. Neuron. 2001;31:453–62.
Lafourcade M, Elezgarai I, Mato S, Bakiri Y, Grandes P, Manzoni OJ. Molecular components and functions of the endocannabinoid system in mouse prefrontal cortex. PLoS One. 2007;2:e709.
Robbe D, Kopf M, Remaury A, Bockaert J, Manzoni OJ. Endogenous cannabinoids mediate long-term synaptic depression in the nucleus accumbens. Proc Natl Acad Sci USA. 2002;99:8384–8.
Bender VA, Bender KJ, Brasier DJ, Feldman DE. Two coincidence detectors for spike timing-dependent plasticity in somatosensory cortex. J Neurosci. 2006;26:4166–77.
Koukouli F, Montmerle M, Aguirre A, De Brito Van Velze M, Peixoto J, Choudhary V, et al. Visual-area-specific tonic modulation of GABA release by endocannabinoids sets the activity and coordination of neocortical principal neurons. Cell Rep. 2022;40:111202.
Bear MF, Huber KM, Warren ST. The mGluR theory of fragile X mental retardation. Trends Neurosci. 2004;27:370–7.
Ronesi JA, Collins KA, Hays SA, Tsai NP, Guo W, Birnbaum SG, et al. Disrupted Homer scaffolds mediate abnormal mGluR5 function in a mouse model of fragile X syndrome. Nat Neurosci. 2012;15:431–40.
Jung KM, Astarita G, Zhu C, Wallace M, Mackie K, Piomelli D. A key role for diacylglycerol lipase-alpha in metabotropic glutamate receptor-dependent endocannabinoid mobilization. Mol Pharmacol. 2007;72:612–21.
Castillo PE, Chiu CQ, Carroll RC. Long-term plasticity at inhibitory synapses. Curr Opin Neurobiol. 2011;21:328–38.
Chevaleyre V, Castillo PE. Heterosynaptic LTD of hippocampal GABAergic synapses: a novel role of endocannabinoids in regulating excitability. Neuron. 2003;38:461–72.
Gomis-Gonzalez M, Busquets-Garcia A, Matute C, Maldonado R, Mato S, Ozaita A. Possible therapeutic doses of cannabinoid type 1 receptor antagonist reverses key alterations in fragile X syndrome mouse model. Genes (Basel). 2016;7:56.
Pirbhoy PS, Jonak CR, Syed R, Argueta DA, Perez PA, Wiley MB, et al. Increased 2-arachidonoyl-sn-glycerol levels normalize cortical responses to sound and improve behaviors in Fmr1 KO mice. J Neurodev Disord. 2021;13:47.
Bakker DB. Fmr1 knockout mice: a model to study fragile X mental retardation. The Dutch-Belgian Fragile X Consortium. Cell. 1994;78:23–33.
Mientjes EJ, Nieuwenhuizen I, Kirkpatrick L, Zu T, Hoogeveen-Westerveld M, Severijnen L, et al. The generation of a conditional Fmr1 knock out mouse model to study Fmrp function in vivo. Neurobiol Dis. 2006;21:549–55.
Castorena CM, Caron A, Michael NJ, Ahmed NI, Arnold AG, Lee J, et al. CB1Rs in VMH neurons regulate glucose homeostasis but not body weight. Am J Physiol Endocrinol Metab. 2021;321:E146–e155.
Harris JA, Hirokawa KE, Sorensen SA, Gu H, Mills M, Ng LL, et al. Anatomical characterization of Cre driver mice for neural circuit mapping and manipulation. Front Neural Circuits. 2014;8:76.
Vong L, Ye C, Yang Z, Choi B, Chua S Jr., Lowell BB. Leptin action on GABAergic neurons prevents obesity and reduces inhibitory tone to POMC neurons. Neuron. 2011;71:142–54.
Zador F, Lenart N, Csibrany B, Santha M, Molnar M, Tuka B, et al. Low dosage of rimonabant leads to anxiolytic-like behavior via inhibiting expression levels and G-protein activity of kappa opioid receptors in a cannabinoid receptor independent manner. Neuropharmacology. 2015;89:298–307.
Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22:659–61.
Pava MJ, den Hartog CR, Blanco-Centurion C, Shiromani PJ, Woodward JJ. Endocannabinoid modulation of cortical up-states and NREM sleep. PLoS One. 2014;9:e88672.
Lu HC, Mackie K. Review of the Endocannabinoid System. Biol Psychiatry Cogn Neurosci Neuroimaging. 2021;6:607–15.
Ogasawara D, Deng H, Viader A, Baggelaar MP, Breman A, den Dulk H, et al. Rapid and profound rewiring of brain lipid signaling networks by acute diacylglycerol lipase inhibition. Proc Natl Acad Sci USA. 2016;113:26–33.
Schurman LD, Carper MC, Moncayo LV, Ogasawara D, Richardson K, Yu L, et al. Diacylglycerol lipase-alpha regulates hippocampal-dependent learning and memory processes in mice. J Neurosci. 2019;39:5949–65.
Liu X, Chen Y, Vickstrom CR, Li Y, Viader A, Cravatt BF, et al. Coordinated regulation of endocannabinoid-mediated retrograde synaptic suppression in the cerebellum by neuronal and astrocytic monoacylglycerol lipase. Sci Rep. 2016;6:35829.
Liu X, Dimidschstein J, Fishell G, Carter AG. Hippocampal inputs engage CCK+ interneurons to mediate endocannabinoid-modulated feed-forward inhibition in the prefrontal cortex. eLife. 2020;9:e55267.
Wilson RI, Nicoll RA. Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature. 2001;410:588–92.
Trettel J, Levine ES. Cannabinoids depress inhibitory synaptic inputs received by layer 2/3 pyramidal neurons of the neocortex. J Neurophysiol. 2002;88:534–9.
Speed HE, Masiulis I, Gibson JR, Powell CM. Increased cortical inhibition in autism-linked neuroligin-3R451C mice is due in part to loss of endocannabinoid signaling. PLoS One. 2015;10:e0140638.
Marsicano G, Lutz B. Expression of the cannabinoid receptor CB1 in distinct neuronal subpopulations in the adult mouse forebrain. Eur J Neurosci. 1999;11:4213–25.
Hedrich J, Angamo EA, Conrad A, Lutz B, Luhmann HJ. Cell type specific impact of cannabinoid receptor signaling in somatosensory barrel map formation in mice. J Comp Neurol. 2020;528:3–13.
Durieux LJA, Gilissen SRJ, Arckens L. Endocannabinoids and cortical plasticity: CB1R as a possible regulator of the excitation/inhibition balance in health and disease. Eur J Neurosci. 2022;55:971–88.
Gorski JA, Talley T, Qiu M, Puelles L, Rubenstein JL, Jones KR. Cortical excitatory neurons and glia, but not GABAergic neurons, are produced in the Emx1-expressing lineage. J Neurosci. 2002;22:6309–14.
Jin SX, Higashimori H, Schin C, Tamashiro A, Men Y, Chiang MSR, et al. Astroglial FMRP modulates synaptic signaling and behavior phenotypes in FXS mouse model. Glia. 2021;69:594–608.
Sinclair D, Oranje B, Razak KA, Siegel SJ, Schmid S. Sensory processing in autism spectrum disorders and Fragile X syndrome-From the clinic to animal models. Neurosci Biobehav Rev. 2017;76:235–53.
Haider B, Duque A, Hasenstaub AR, McCormick DA. Neocortical network activity in vivo is generated through a dynamic balance of excitation and inhibition. J Neurosci. 2006;26:4535–45.
Lemieux M, Chauvette S, Timofeev I. Neocortical inhibitory activities and long-range afferents contribute to the synchronous onset of silent states of the neocortical slow oscillation. J Neurophysiol. 2015;113:768–79.
Sanchez-Vives MV, Mattia M, Compte A, Perez-Zabalza M, Winograd M, Descalzo VF, et al. Inhibitory Modulation of Cortical Up States. J Neurophysiol. 2010;104:1314–24.
Mann EO, Kohl MM, Paulsen O. Distinct roles of GABAA and GABAB receptors in balancing and terminating persistent cortical activity. J Neurosci. 2009;29:7513–8.
Rey AA, Purrio M, Viveros M-P, Lutz B. Biphasic effects of cannabinoids in anxiety responses: CB1 and GABAB receptors in the balance of GABAergic and glutamatergic neurotransmission. Neuropsychopharmacology. 2012;37:2624–34.
Jonak CR, Pedapati EV, Schmitt LM, Assad SA, Sandhu MS, DeStefano L, et al. Baclofen-associated neurophysiologic target engagement across species in fragile X syndrome. J Neurodev Disord. 2022;14:52.
Neske GT, Connors BW. Distinct roles of SOM and VIP interneurons during cortical up states. Front Neural Circuits. 2016;10:52.
Zucca S, D’Urso G, Pasquale V, Vecchia D, Pica G, Bovetti S, et al. An inhibitory gate for state transition in cortex. eLife. 2017;6:e26177.
Bodor AL, Katona I, Nyíri G, Mackie K, Ledent C, Hájos N, et al. Endocannabinoid signaling in rat somatosensory cortex: laminar differences and involvement of specific interneuron types. J Neurosci. 2005;25:6845–56.
Hill EL, Gallopin T, Férézou I, Cauli B, Rossier J, Schweitzer P, et al. Functional CB1 receptors are broadly expressed in neocortical GABAergic and glutamatergic neurons. J Neurophysiol. 2007;97:2580–9.
Wedzony K, Chocyk A. Cannabinoid CB1 receptors in rat medial prefrontal cortex are colocalized with calbindin- but not parvalbumin- and calretinin-positive GABA-ergic neurons. Pharmacol Rep. 2009;61:1000–7.
Katona I, Sperlagh B, Sik A, Kafalvi A, Vizi ES, Mackie K, et al. Presynaptically located CB1 cannabinoid receptors regulate GABA release from axon terminals of specific hippocampal interneurons. J Neurosci. 1999;19:4544–58.
Neu A, Földy C, Soltesz I. Postsynaptic origin of CB1-dependent tonic inhibition of GABA release at cholecystokinin-positive basket cell to pyramidal cell synapses in the CA1 region of the rat hippocampus. J Physiol. 2007;578:233–47.
Roberto M, Cruz M, Bajo M, Siggins GR, Parsons LH, Schweitzer P. The endocannabinoid system tonically regulates inhibitory transmission and depresses the effect of ethanol in central amygdala. Neuropsychopharmacology. 2010;35:1962–72.
Aloisi E, Le Corf K, Dupuis J, Zhang P, Ginger M, Labrousse V, et al. Altered surface mGluR5 dynamics provoke synaptic NMDAR dysfunction and cognitive defects in Fmr1 knockout mice. Nat Commun. 2017;8:1103.
Omiya Y, Uchigashima M, Konno K, Yamasaki M, Miyazaki T, Yoshida T, et al. VGluT3-expressing CCK-positive basket cells construct invaginating synapses enriched with endocannabinoid signaling proteins in particular cortical and cortex-like amygdaloid regions of mouse brains. J Neurosci. 2015;35:4215–28.
Skosnik PD, Cortes-Briones JA, Hajós M. It’s all in the rhythm: the role of cannabinoids in neural oscillations and psychosis. Biol Psychiatry. 2016;79:568–77.
Syed SA, Schnakenberg Martin AM, Cortes-Briones JA, Skosnik PD. The relationship between cannabinoids and neural oscillations: how cannabis disrupts sensation, perception, and cognition. Clin EEG Neurosci. 2023;54:359–69.
Cortes-Briones J, Skosnik PD, Mathalon D, Cahill J, Pittman B, Williams A, et al. Δ9-THC disrupts gamma (γ)-band neural oscillations in humans. Neuropsychopharmacology. 2015;40:2124–34.
Whissell PD, Cajanding JD, Fogel N, Kim JC. Comparative density of CCK- and PV-GABA cells within the cortex and hippocampus. Front Neuroanat. 2015;9:124.
Eggan SM, Lewis DA. Immunocytochemical distribution of the cannabinoid CB1 receptor in the primate neocortex: a regional and laminar analysis. Cereb Cortex. 2006;17:175–91.
Skosnik PD, D’Souza DC, Steinmetz AB, Edwards CR, Vollmer JM, Hetrick WP, et al. The effect of chronic cannabinoids on broadband EEG neural oscillations in humans. Neuropsychopharmacology. 2012;37:2184–93.
Foldy C, Malenka RC, Sudhof TC. Autism-associated neuroligin-3 mutations commonly disrupt tonic endocannabinoid signaling. Neuron. 2013;78:498–509.
Anderson GR, Aoto J, Tabuchi K, Földy C, Covy J, Yee AX, et al. β-Neurexins control neural circuits by regulating synaptic endocannabinoid signaling. Cell. 2015;162:593–606.
Acknowledgements
We would like to acknowledge Jacob E. Bowles and Christopher Williams for technical assistance with genotyping and Dr. Laurent Gautron for assistance with RNAscope. This work was supported by NIH grants U54HD082008 and U54HD104461 (KMH, JRG, DKB) and P30DK127984 (UTSW Metabolic Core).
Author information
Authors and Affiliations
Contributions
DG, CRJ, MB, GM, KC, and SAA performed experiments and data analysis. JRG developed software for data acquisition and analysis and performed data analysis. JRG, DKB, and KMH contributed to experimental design, data analysis, wrote the manuscript and obtained funding for the project.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics Approval
All experimental procedures have been approved by the Institutional Animal Care and Use Committees at UT Southwestern (Protocol #’s 2015-101252; 2017-101986) or UC Riverside (Protocol #: #20190015) and conducted in full accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gonzalez, D., Jonak, C., Bernabucci, M. et al. Enhanced CB1 receptor function in GABAergic neurons mediates hyperexcitability and impaired sensory-driven synchrony of cortical circuits in Fragile X Syndrome model mice. Mol Psychiatry (2025). https://doi.org/10.1038/s41380-025-03366-6
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41380-025-03366-6