Abstract
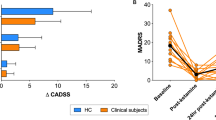
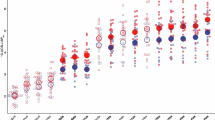
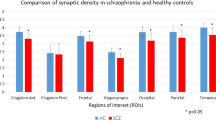
Ketamine was shown to promote synaptogenesis, which is thought to account for its antidepressant effects through the restoration of lost synaptic connections observed in depression. PET imaging using 11C-UCB-J, a radiotracer targeting the synaptic vesicle protein 2 A (SV2A), was investigated as a translational method to monitor ketamine-induced changes in synaptic density in the corticosterone (CORT) mouse model of anxiety/depression. Male CORT and healthy control mice received either a single dose of ketamine (10 mg/kg, i.p) or a repeated-dose regimen (3 doses in total). Brain PET imaging was performed either 24 h after the single dose or 1 or 3 weeks after the repeated-dose regimen to estimate the binding of 11C-UCB-J in each condition. A global decrease in the binding of 11C-UCB-J was observed in CORT mice compared to control mice, indicating synaptic loss. In CORT mice, behavioral experiments showed antidepressant effects of ketamine 24 h after a single dose, although no significant changes in 11C-UCB-J binding could be observed at this time point. Three weeks after the repeated dose regimen, ketamine restored the binding of 11C-UCB-J in CORT mice to the level of age-matched untreated healthy controls. The reversal of synaptic loss was associated with delayed antidepressant effects in behavioural tests. Ex vivo expression of SV2A protein measured under different conditions was strongly correlated with the in vivo binding of 11C-UCB-J and the postsynaptic marker PSD95. These results support the molecular interpretation of SV2A PET imaging to monitor drug-induced synaptogenesis as a determinant of antidepressant efficacy from a translational perspective.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data supporting this study’s findings are available from the corresponding author upon reasonable request.
References
Depressive disorder (depression). https://www.who.int/news-room/fact-sheets/detail/depression. Accessed 3 February 2025.
Yavi M, Lee H, Henter ID, Park LT, Zarate CA. Ketamine treatment for depression: a review. Discov Ment Health. 2022;2:9.
Zanos P, Gould TD. Mechanisms of ketamine action as an antidepressant. Mol Psychiatry. 2018;23:801–11.
Eduardo T-Q, Angela A, Mateo L, Melanie L-Z, Valentina P-F, David C, et al. Ketamine for resistant depression: a scoping review. Actas Esp Psiquiatr. 2022;50:144–59.
Krystal JH, Kavalali ET, Monteggia LM. Ketamine and rapid antidepressant action: new treatments and novel synaptic signaling mechanisms. Neuropsychopharmacol. 2024;49:41–50.
Duman RS, Aghajanian GK. Synaptic dysfunction in depression: potential therapeutic targets. Science. 2012;338:68–72.
Duric V, Banasr M, Stockmeier CA, Simen AA, Newton SS, Overholser JC, et al. Altered expression of synapse and glutamate related genes in post-mortem hippocampus of depressed subjects. Int J Neuropsychopharmacol. 2013;16:69–82.
Kang HJ, Voleti B, Hajszan T, Rajkowska G, Stockmeier C, Licznerski P, et al. Decreased expression of synapse-related genes and loss of synapses in major depressive disorder. Nat Med. 2012;18:1413–7.
Serretti A. The pharmacological management of treatment-resistant depression: what does the future hold? Expert Opin Pharmacother. 2023;24:1923–5.
Li N, Lee B, Liu R-J, Banasr M, Dwyer JM, Iwata M, et al. mTOR-Dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959–64.
Samuels BA, Leonardo ED, Gadient R, Williams A, Zhou J, David DJ, et al. MODELING TREATMENT-RESISTANT DEPRESSION. Neuropharmacology. 2011;61:408–13.
Howes O, Marcinkowska J, Turkheimer FE, Carr R Synaptic changes in psychiatric and neurological disorders: state-of-the art of in vivo imaging. Neuropsychopharmacol. 2024;50:164–83.
Rossi R, Arjmand S, Bærentzen SL, Gjedde A, Landau AM. Synaptic vesicle glycoprotein 2A: features and functions. Front Neurosci. 2022;16:864514.
Mendoza-Torreblanca JG, Vanoye-Carlo A, Phillips-Farfán BV, Carmona-Aparicio L, Gómez-Lira G. Synaptic vesicle protein 2A: basic facts and role in synaptic function. Eur J Neurosci. 2013;38:3529–39.
Finnema SJ, Nabulsi NB, Eid T, Detyniecki K, Lin S, Chen M-K, et al. Imaging synaptic density in the living human brain. Sci Transl Med. 2016;8:348ra96–348ra96.
Shanaki Bavarsad M, Spina S, Oehler A, Allen IE, Suemoto CK, Leite REP, et al. Comprehensive mapping of synaptic vesicle protein 2A (SV2A) in health and neurodegenerative diseases: a comparative analysis with synaptophysin and ground truth for PET-imaging interpretation. Acta Neuropathol. 2024;148:58.
Heurling K, Ashton NJ, Leuzy A, Zimmer ER, Blennow K, Zetterberg H, et al. Synaptic vesicle protein 2A as a potential biomarker in synaptopathies. Mol Cell Neurosci. 2019;97:34–42.
Becker G, Dammicco S, Bahri MA, Salmon E. The rise of synaptic density PET imaging. Molecules. 2020;25:2303.
Holmes SE, Scheinost D, Finnema SJ, Naganawa M, Davis MT, DellaGioia N, et al. Lower synaptic density is associated with depression severity and network alterations. Nat Commun. 2019;10:1529.
Holmes SE, Finnema SJ, Naganawa M, DellaGioia N, Holden D, Fowles K, et al. Imaging the effect of ketamine on synaptic density (SV2A) in the living brain. Mol Psychiatry. 2022;27:2273–81.
von Mücke-Heim I-A, Urbina-Treviño L, Bordes J, Ries C, Schmidt MV, Deussing JM. Introducing a depression-like syndrome for translational neuropsychiatry: a plea for taxonomical validity and improved comparability between humans and mice. Mol Psychiatry. 2023;28:329–40.
Agasse F, Mendez-David I, Christaller W, Carpentier R, Braz BY, David DJ, et al. Chronic corticosterone elevation suppresses adult hippocampal neurogenesis by hyperphosphorylating huntingtin. Cell Rep. 2020;32:107865.
Nomoto K, Kansaku K. Chronic corticosterone deteriorates latrine and nesting behaviours in mice. R Soc Open Sci. 2023;10:220718.
Wang H, Wang X, Wang H, Shao S, Zhu J. Chronic corticosterone administration-induced mood disorders in laboratory rodents: features, mechanisms, and research perspectives. Int J Mol Sci. 2024;25:11245.
David DJ, Samuels BA, Rainer Q, Wang J-W, Marsteller D, Mendez I, et al. Neurogenesis-dependent and -independent effects of fluoxetine in an animal model of anxiety/depression. Neuron. 2009;62:479–93.
Hodes GE, Hill-Smith TE, Lucki I. Fluoxetine treatment induces dose dependent alterations in depression associated behavior and neural plasticity in female mice. Neurosci Lett. 2010;484:12–16.
Corvo C, Mendez-David I, Goutal S, Saba W, Bottlaender M, Caillé F, et al. Synaptic vesicle 2A (SV2A) positron emission tomography (PET) imaging as a marker of therapeutic response in a mouse model of depression. ACS Pharmacol Transl Sci. 2025;8:339–45.
Xu S, Yao X, Li B, Cui R, Zhu C, Wang Y, et al. Uncovering the underlying mechanisms of ketamine as a novel antidepressant. Front Pharmacol. 2021;12:740996.
Mendez-David I, Guilloux J-P, Papp M, Tritschler L, Mocaer E, Gardier AM, et al. S 47445 produces antidepressant- and anxiolytic-like effects through neurogenesis dependent and independent mechanisms. Front Pharmacol. 2017;8:462.
Moy SS, Nadler JJ, Perez A, Barbaro RP, Johns JM, Magnuson TR, et al. Sociability and preference for social novelty in five inbred strains: an approach to assess autistic-like behavior in mice. Genes Brain Behav. 2004;3:287–302.
Milicevic Sephton S, Miklovicz T, Russell JJ, Doke A, Li L, Boros I, et al. Automated radiosynthesis of [11C]UCB‐J for imaging synaptic density by positron emission tomography. J Labelled Comp Radiopharm. 2020;63:151–8.
Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, et al. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab. 2007;27:1533–9.
Bertoglio D, Verhaeghe J, Miranda A, Kertesz I, Cybulska K, Korat Š, et al. Validation and noninvasive kinetic modeling of [11C]UCB-J PET imaging in mice. J Cereb Blood Flow Metab. 2020;40:1351–62.
Logan J, Fowler JS, Volkow ND, Wolf AP, Dewey SL, Schlyer DJ, et al. Graphical analysis of reversible radioligand binding from time—activity measurements applied to [N-11C-Methyl]-(−)-Cocaine PET studies in human subjects. J Cereb Blood Flow Metab. 1990;10:740–7.
Ma S, Chen M, Jiang Y, Xiang X, Wang S, Wu Z, et al. Sustained antidepressant effect of ketamine through NMDAR trapping in the LHb. Nature. 2023;622:802–9.
Gencturk S, Unal G. Rodent tests of depression and anxiety: Construct validity and translational relevance. Cogn Affect Behav Neurosci. 2024;24:191–224.
Yoshioka T, Yamada D, Hagiwara A, Kajino K, Iio K, Saitoh T, et al. Delta opioid receptor agonists activate PI3K–mTORC1 signaling in parvalbumin-positive interneurons in mouse infralimbic prefrontal cortex to exert acute antidepressant-like effects. Mol Psychiatry. 2025;30:2038–48.
Kumar A, Scarpa M, Nordberg A. Tracing synaptic loss in Alzheimer’s brain with SV2A PET‐tracer UCB‐J. Alzheimers Dement. 2024;20:2589–605.
Liston C, Miller MM, Goldwater DS, Radley JJ, Rocher AB, Hof PR, et al. Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. J Neurosci. 2006;26:7870–4.
Popoli M, Yan Z, McEwen B, Sanacora G. The stressed synapse: the impact of stress and glucocorticoids on glutamate transmission. Nat Rev Neurosci. 2011;13:22–37.
Sanacora G, Treccani G, Popoli M. Towards a glutamate hypothesis of depression: an emerging frontier of neuropsychopharmacology for mood disorders. Neuropharmacology. 2012;62:63–77.
Asch RH, Abdallah CG, Carson RE, Esterlis I. Challenges and rewards of in vivo synaptic density imaging, and its application to the study of depression. Neuropsychopharmacology. 2024;50:153–63.
Hasler G, Northoff G. Discovering imaging endophenotypes for major depression. Mol Psychiatry. 2011;16:604–19.
Wang C, Lan X, Liu W, Zhan Y, Zheng W, Chen X, et al. Non-improvement predicts subsequent non-response to repeated-dose intravenous ketamine for depression: a re-analysis of a 2-week open-label study in patients with unipolar and bipolar depression. Transl Psychiatry. 2024;14:324.
Fraga DB, Camargo A, Olescowicz G, Padilha DA, Mina F, Budni J, et al. Ketamine, but not fluoxetine, rapidly rescues corticosterone-induced impairments on glucocorticoid receptor and dendritic branching in the hippocampus of mice. Metab Brain Dis. 2021;36:2223–33.
Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng P, et al. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature. 2011;475:91–95.
Lisek M, Ferenc B, Studzian M, Pulaski L, Guo F, Zylinska L, et al. Glutamate deregulation in ketamine-induced psychosis—a potential role of PSD95, NMDA receptor and PMCA interaction. Front Cell Neurosci. 2017;11:181.
Yoo K-S, Lee K, Oh J-Y, Lee H, Park H, Park YS, et al. Postsynaptic density protein 95 (PSD-95) is transported by KIF5 to dendritic regions. Mol Brain. 2019;12:97.
Zhang Y, Wei C-K, Wang P, Zheng L-C, Cheng Y, Ren Z-H, et al. S-ketamine alleviates depression-like behavior and hippocampal neuroplasticity in the offspring of mice that experience prenatal stress. Sci Rep. 2024;14:26929.
Huang B, Li X, Zheng Y, Mai Y, Zhang Z. Effects of esketamine on depression-like behavior and dendritic spine plasticity in the prefrontal cortex neurons of spared nerve injury-induced depressed mice. Braz J Med Biol Res. 2024;57:e13736.
Moda-Sava RN, Murdock MH, Parekh PK, Fetcho RN, Huang BS, Huynh TN, et al. Sustained rescue of prefrontal circuit dysfunction by antidepressant-induced spine formation. Science. 2019;364:eaat8078.
Wenzel M, Leunig A, Han S, Peterka DS, Yuste R. Prolonged anesthesia alters brain synaptic architecture. Proc Natl Acad Sci. 2021;118:e2023676118.
Johansen A, Armand S, Plavén-Sigray P, Nasser A, Ozenne B, Petersen IN, et al. Effects of escitalopram on synaptic density in the healthy human brain: a randomized controlled trial. Mol Psychiatry. 2023;28:4272–9.
Rayan NA, Kumar V, Aow J, Rastegar N, Lim MGL, O’Toole N, et al. Integrative multi-omics landscape of fluoxetine action across 27 brain regions reveals global increase in energy metabolism and region-specific chromatin remodelling. Mol Psychiatry. 2022;27:4510–25.
Page CE, Epperson CN, Novick AM, Duffy KA, Thompson SM. Beyond the serotonin deficit hypothesis: communicating a neuroplasticity framework of major depressive disorder. Mol Psychiatry. 2024;29:3802–13.
Acknowledgements
We thank Maud Goislard and Sophie Amargier-Barrial for their technical support during the preclinical study. We also thank Stephane Demphel and Françoise Hinnen for producing 11C-UCB-J. This work is funded by the French National Agency for Research (Grant ANR-22-CE17-0015) and was performed on a platform member of the France Life Imaging network (grant ANR-11-INBS-0006).
Author information
Authors and Affiliations
Contributions
RC, CL, DJD, and NT obtained funding and resources to conduct the study. CC, CL, DJD, IMD, SG, and NT conceived and planned the study. CC and IMD have developed and applied the model and behavioral assessments. FC has developed and supervised the synthesis of 11C-UCB-J. CC, SG, and SL conducted the PET imaging experiments and analyzed the data. The results were interpreted by FC, WS, SG, NT, CL, EC, VL, MB, RC, DJD, and IMD. CC and NT wrote the manuscript and made the figures. All authors revised and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
These authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Corvo, C., Goutal, S., Mendez-David, I. et al. Quantitative monitoring of ketamine’s impact on synaptic density using 11C-UCB-J PET imaging in the corticosterone mouse model of anxiety/depression. Mol Psychiatry (2025). https://doi.org/10.1038/s41380-025-03369-3
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41380-025-03369-3