Abstract
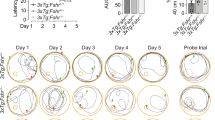
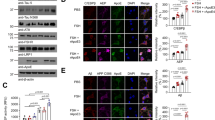
Epidemiologic evidence links follicle–stimulating hormone (FSH), a pituitary glycoprotein that rises during menopause, to memory loss, fat accumulation, and bone loss. We and others have shown that the attenuation of FSH signaling, either genetically or pharmacologically, prevents memory loss, fat accrual, and bone loss in multiple mouse models. Here, we investigated whether the genetic depletion of the FSH receptor (Fshr) affects recognition memory, body composition, and bone mineral density (BMD) in two AD mouse models. We generated male and female 3xTg and APP-KI mice carrying the Fshr+/+, Fshr+/−, and Fshr−/− genotypes. Recognition memory was evaluated using the Novel Object Recognition (NOR) test. Body composition (fat, lean, and total mass) and site–specific bone mineral density (femur, tibia, L3–L5 spine) measurements were made using quantitative nuclear magnetic resonance (qNMR) and dual-energy X-ray absorptiometry (DXA), respectively, at two time points. Given that female Fshr−/− genotypes are otherwise hypogonadal, they were implanted with 17β–estradiol pellets at 8–12 weeks of age to normalize serum estrogen. At the early time point, the deficit in recognition memory was rescued in female 3xTg;Fshr−/− and APP-KI;Fshr−/− mice, but not in male mice. Likewise, female, but not male 3xTg;Fshr−/− mice showed reduced fat mass at both the early and later time points, but without changes in total body mass. In contrast, in the APP-KI cohort, both female and male Fshr-/- mice showed reduced fat mass at the early, but not the late time point. DXA revealed that female, but not male APP-KI;Fshr−/− mice showed progressive increases with time in BMDs in tibiae, femora, and vertebrae, which were either statistically significant or approached significance. This phenotype was not observed on the 3xTg background. These studies constitute the first report for time– and strain–dependent effects of global Fshr depletion in the same mouse, setting the stage for the simultaneous prevention, using a single therapeutic, of three disorders of public health magnitude—Alzheimer’s disease, obesity and osteoporosis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
Data are available from the corresponding authors on reasonable request.
References
Abe E, Marians RC, Yu W, Wu XB, Ando T, Li Y, et al. TSH is a negative regulator of skeletal remodeling. Cell. 2003;115:151–62.
Sun L, Lizneva D, Ji Y, Colaianni G, Hadelia E, Gumerova A, et al. Oxytocin regulates body composition. Proc. Natl Acad. Sci. USA. 2019;116:26808–15.
Sun L, Peng Y, Sharrow AC, Iqbal J, Zhang Z, Papachristou DJ, et al. FSH directly regulates bone mass. Cell. 2006;125:247–60.
Zaidi M, Yuen T, Sun L, Davies TF, Zallone A, Blair HC. The Pituitary‐Bone Connection. Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism. Hoboken NJ: John Wiley & Sons, Inc., 2013, pp 969–77.
Liu P, Ji Y, Yuen T, Rendina-Ruedy E, DeMambro VE, Dhawan S, et al. Blocking FSH induces thermogenic adipose tissue and reduces body fat. Nature. 2017;546:107–12.
Xiong J, Kang SS, Wang Z, Liu X, Kuo TC, Korkmaz F, et al. FSH blockade improves cognition in mice with Alzheimer’s disease. Nature. 2022;603:470–6.
Xiong J, Kang SS, Wang M, Wang Z, Xia Y, Liao J, et al. FSH and ApoE4 contribute to Alzheimer’s disease-like pathogenesis via C/EBPbeta/delta-secretase in female mice. Nat. Commun. 2023;14:6577.
Korkmaz F, Sims S, Sen F, Sultana F, Laurencin V, Cullen L, et al. Gene-dose-dependent reduction of Fshr expression improves spatial memory deficits in Alzheimer’s mice. Mol. Psychiatry. 2025;30:2119–26.
Greendale GA, Huang MH, Wight RG, Seeman T, Luetters C, Avis NE, et al. Effects of the menopause transition and hormone use on cognitive performance in midlife women. Neurology. 2009;72:1850–7.
Greendale GA, Wight RG, Huang MH, Avis N, Gold EB, Joffe H, et al. Menopause-associated symptoms and cognitive performance: results from the study of women’s health across the nation. Am. J. Epidemiol. 2010;171:1214–24.
Randolph JF Jr., Sowers M, Gold EB, Mohr BA, Luborsky J, Santoro N, et al. Reproductive hormones in the early menopausal transition: relationship to ethnicity, body size, and menopausal status. J. Clin. Endocrinol. Metab. 2003;88:1516–22.
Greendale GA, Sowers M, Han W, Huang MH, Finkelstein JS, Crandall CJ, et al. Bone mineral density loss in relation to the final menstrual period in a multiethnic cohort: results from the Study of Women’s Health Across the Nation (SWAN). J. Bone Min. Res. 2012;27:111–8.
Greendale GA, Sternfeld B, Huang M, Han W, Karvonen-Gutierrez C, Ruppert K, et al. Changes in body composition and weight during the menopause transition. JCI Insight. 2019;4:e124865.
Sowers M, Jannausch ML, Gross M, Karvonen-Gutierrez CA, Palmieri RM, Crutchfield M, et al. Performance-based physical functioning in African-American and Caucasian women at midlife: considering body composition, quadriceps strength, and knee osteoarthritis. Am. J. Epidemiol. 2006;163:950–8.
Sowers M, Zheng H, Tomey K, Karvonen-Gutierrez C, Jannausch M, Li X, et al. Changes in body composition in women over six years at midlife: ovarian and chronological aging. J. Clin. Endocrinol. Metab. 2007;92:895–901.
Sowers MR, Finkelstein JS, Ettinger B, Bondarenko I, Neer RM, Cauley JA, et al. The association of endogenous hormone concentrations and bone mineral density measures in pre- and perimenopausal women of four ethnic groups: SWAN. Osteoporos. Int. 2003;14:44–52.
Sowers MR, Greendale GA, Bondarenko I, Finkelstein JS, Cauley JA, Neer RM, et al. Endogenous hormones and bone turnover markers in pre- and perimenopausal women: SWAN. Osteoporos. Int. 2003;14:191–7.
Sowers MR, Jannausch M, McConnell D, Little R, Greendale GA, Finkelstein JS, et al. Hormone predictors of bone mineral density changes during the menopausal transition. J. Clin. Endocrinol. Metab. 2006;91:1261–7.
Ji Y, Liu P, Yuen T, Haider S, He J, Romero R, et al. Epitope-specific monoclonal antibodies to FSHbeta increase bone mass. Proc. Natl Acad. Sci. USA. 2018;115:2192–7.
Zhu LL, Blair H, Cao J, Yuen T, Latif R, Guo L, et al. Blocking antibody to the beta-subunit of FSH prevents bone loss by inhibiting bone resorption and stimulating bone synthesis. Proc. Natl Acad. Sci. USA. 2012;109:14574–9.
Gera S, Kuo TC, Gumerova AA, Korkmaz F, Sant D, DeMambro V, et al. FSH-blocking therapeutic for osteoporosis. Elife. 2022;11:e78022.
Gera S, Sant D, Haider S, Korkmaz F, Kuo TC, Mathew M, et al. First-in-class humanized FSH blocking antibody targets bone and fat. Proc. Natl Acad. Sci. USA. 2020;117:28971–9.
Han X, Guan Z, Xu M, Zhang Y, Yao H, Meng F, et al. A novel follicle-stimulating hormone vaccine for controlling fat accumulation. Theriogenology. 2020;148:103–11.
Geng W, Yan X, Du H, Cui J, Li L, Chen F. Immunization with FSHbeta fusion protein antigen prevents bone loss in a rat ovariectomy-induced osteoporosis model. Biochem. Biophys. Res. Commun. 2013;434:280–6.
Pallapati AR, Korkmaz F, Rojekar S, Sims S, Misra A, Gimenez-Roig J, et al. Efficacy and safety of a therapeutic humanized FSH-blocking antibody in obesity and Alzheimer’s disease models. J. Clin. Invest. 2025;135:e182702.
Randolph JF Jr., Sowers M, Bondarenko I, Gold EB, Greendale GA, Bromberger JT, et al. The relationship of longitudinal change in reproductive hormones and vasomotor symptoms during the menopausal transition. J. Clin. Endocrinol. Metab. 2005;90:6106–12.
Janelsins MC, Mastrangelo MA, Oddo S, LaFerla FM, Federoff HJ, Bowers WJ. Early correlation of microglial activation with enhanced tumor necrosis factor-alpha and monocyte chemoattractant protein-1 expression specifically within the entorhinal cortex of triple transgenic Alzheimer’s disease mice. J. Neuroinflammation. 2005;2:23.
Iqbal J, Sun L, Kumar TR, Blair HC, Zaidi M. Follicle-stimulating hormone stimulates TNF production from immune cells to enhance osteoblast and osteoclast formation. Proc. Natl Acad. Sci. USA. 2006;103:14925–30.
Jiao Y, Sun J, Li Y, Zhao J, Shen J. Association between adiposity and bone mineral density in adults: insights from a national survey analysis. Nutrients. 2023;15:3492.
Randolph JF Jr, Zheng H, Sowers MR, Crandall C, Crawford S, Gold EB, et al. Change in follicle-stimulating hormone and estradiol across the menopausal transition: effect of age at the final menstrual period. J. Clin. Endocrinol. Metab. 2011;96:746–54.
Sims S, Barak O, Ryu V, Miyashita S, Kannangara H, Korkmaz F, et al. Absent LH signaling rescues the anxiety phenotype in aging female mice. Mol. Psychiatry. 2023;28:3324–31.
Leger M, Quiedeville A, Bouet V, Haelewyn B, Boulouard M, Schumann-Bard P, et al. Object recognition test in mice. Nat. Protoc. 2013;8:2531–7.
Clark RE, Martin SJ. Interrogating rodents regarding their object and spatial memory. Curr. Opin. Neurobiol. 2005;15:593–8.
Ennaceur A, Delacour J. A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behav. Brain Res. 1988;31:47–59.
Bevins RA, Besheer J. Object recognition in rats and mice: a one-trial non-matching-to-sample learning task to study ‘recognition memory. Nat. Protoc. 2006;1:1306–11.
Taglialatela G, Hogan D, Zhang WR, Dineley KT. Intermediate- and long-term recognition memory deficits in Tg2576 mice are reversed with acute calcineurin inhibition. Behav. Brain Res. 2009;200:95–99.
Acknowledgements
MZ, CJR, TY, KAG, VR and WZ acknowledge the support from the National Institutes of Health (NIH), namely R01 AG074092, U01 AG073148 and R01 AG071870 to MZ, and TY, U19 AG060917 to MZ and CJR, RF1 AG093773 to KAG and MZ, and R61 AG094602 to TY, KAG, VR, WZ and MZ. MZ and TY also acknowledge the Hevolution Foundation for support.
Author information
Authors and Affiliations
Contributions
Conceptualization: TY, MZ. Supervision: TF, TY, MZ. Scientific input: KG, CJR, VR, WZ. Neurobehavioral studies: SS, TF. Body Composition Assessment: UC, AS, AL, JGR, ZT, SP, SS. Data analysis: UC, TF, DV, FK, SS, FS, VL, OM, ARP, SR, TY. Animal maintenance: UC, TL, FK, DV. Methodology: TF, TY. Quality assurance: AG, GP, GB, AM, SH, YW, OB, DL, Manuscript preparation: UC, SS, TF, TY, MZ. Funding acquisition: TY, CJR, MZ, KG, VR, WZ.
Corresponding authors
Ethics declarations
Competing interests
MZ is an inventor on issued patents on inhibiting FSH for the prevention and treatment of osteoporosis and obesity (U.S. patents 8,435,948 and 11,034,761). MZ is also an inventor on a patent application on the composition and use of humanized monoclonal anti-FSH antibodies and is a co–inventor of a pending patent on the use of FSH as a target for preventing Alzheimer disease. MZ, SR and TY are co–inventors on a pending patent relating to the ultra–high formulation of an FSH-blocking antibody. DL, MZ and TY are co–inventors of a pending patent relating to LH and body composition. These patents are owned by Icahn School of Medicine at Mount Sinai (ISMMS), and the inventors and co–inventors would be recipients of royalties, per institutional policy. MZ also consults for several financial platforms, including Gerson Lehman Group and Guidepoint, on drugs for osteoporosis and genetic bone diseases. The other authors declare no competing interests.
Ethics approval
All methods were performed in accordance with the relevant guidelines and regulations. Animal handling and use were compliant with the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals, and approved by the Icahn School of Medicine at Mount Sinai Institutional Animal Care and Use Committee (IACUC Approval # 2018-0047).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
41380_2025_3424_MOESM5_ESM.pdf
Figure S3: No Side Preference Observed During Training Trial Prior to Novel Object Recognition Test in APP-KI and 3xTg Female Fshr Mutants.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cheliadinova, U., Sims, S., Korkmaz, F. et al. Fshr gene depletion prevents recognition memory loss, fat accrual and bone loss in Alzheimer’s mice. Mol Psychiatry (2025). https://doi.org/10.1038/s41380-025-03424-z
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41380-025-03424-z