Abstract
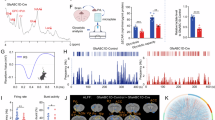
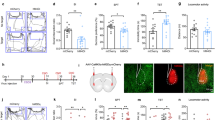
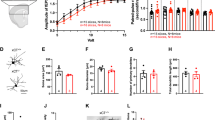
Multiple lines of evidence, including clinical and preclinical, associate depression with dysregulated glutamate homeostasis in the central nervous system. The astrocyte-enriched cysteine/glutamate antiporter, xCT, plays a pivotal role in maintaining this homeostasis, and its dysfunction is linked to depressive-like behaviors induced by chronic stress. However, the exact mechanism through which chronic stress results in xCT dysfunction remains poorly understood. In this study, we demonstrate that chronic social defeat stress (CSDS) downregulates xCT expression in the prelimbic cortex (PL), a key cortical region involved in the aetiology of depression, through astroglial kappa opioid receptor (KOR)-dependent, p38 MAPK-mediated upregulation of miR-3084-5p expression, which in turn downregulates xCT expression by inhibiting mRNA that encodes xCT. Knockdown of KORs as well as overexpression of xCT in the PL astrocytes significantly attenuates CSDS-induced depressive-like behaviors. Blockade of p38 MAPK activity or suppression of miR-3084-5p function restores xCT expression and alleviates depressive-like behaviors. Together, this study reveals a novel mechanism by which chronic stress deregulates glutamate homeostasis and consequently results in depression.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding authors upon reasonable request.
References
Monroe SM, Harkness KL. Major depression and its recurrences: life course matters. Annu Rev Clin Psychol. 2022;18:329–57.
Ferrari AJ, Baxter AJ, Whiteford HA. A systematic review of the global distribution and availability of prevalence data for bipolar disorder. J Affect Disord. 2011;134:1–13.
Sanacora G, Zarate CA, Krystal JH, Manji HK. Targeting the glutamatergic system to develop novel, improved therapeutics for mood disorders. Nat Rev Drug Discov. 2008;7:426–37.
Niciu MJ, Kelmendi B, Sanacora G. Overview of glutamatergic neurotransmission in the nervous system. Pharmacol Biochem Behav. 2012;100:656–64.
Popoli M, Yan Z, McEwen BS, Sanacora G. The stressed synapse: the impact of stress and glucocorticoids on glutamate transmission. Nat Rev Neurosci. 2011;13:22–37.
Zarate CA Jr, Singh JB, Quiroz JA, De Jesus G, Denicoff KK, Luckenbaugh DA, et al. A double-blind, placebo-controlled study of memantine in the treatment of major depression. Am J Psychiatry. 2006;163:153–5.
Zarate C Jr, Machado-Vieira R, Henter I, Ibrahim L, Diazgranados N, Salvadore G. Glutamatergic modulators: the future of treating mood disorders? Harv Rev Psychiatry. 2010;18:293–303.
Nasca C, Xenos D, Barone Y, Caruso A, Scaccianoce S, Matrisciano F, et al. L-acetylcarnitine causes rapid antidepressant effects through the epigenetic induction of mGlu2 receptors. Proc Natl Acad Sci USA. 2013;110:4804–9.
Ottestad-Hansen S, Hu QX, Follin-Arbelet VV, Bentea E, Sato H, Massie A, et al. The cystine-glutamate exchanger (xCT, Slc7a11) is expressed in significant concentrations in a subpopulation of astrocytes in the mouse brain. Glia. 2018;66:951–70.
Pajarillo E, Rizor A, Lee J, Aschner M, Lee E. The role of astrocytic glutamate transporters GLT-1 and GLAST in neurological disorders: potential targets for neurotherapeutics. Neuropharmacology. 2019;161:107559.
Baker DA, Shen H, Kalivas PW. Cystine/glutamate exchange serves as the source for extracellular glutamate: modifications by repeated cocaine administration. Amino acids. 2002;23:161–2.
Lewerenz J, Hewett SJ, Huang Y, Lambros M, Gout PW, Kalivas PW, et al. The cystine/glutamate antiporter system x(c)(-) in health and disease: from molecular mechanisms to novel therapeutic opportunities. Antioxid redox Signal. 2013;18:522–55.
Reissner KJ, Gipson CD, Tran PK, Knackstedt LA, Scofield MD, Kalivas PW. Glutamate transporter GLT-1 mediates N-acetylcysteine inhibition of cocaine reinstatement. Addiction Biol. 2015;20:316–23.
Nasca C, Bigio B, Zelli D, de Angelis P, Lau T, Okamoto M, et al. Role of the astroglial glutamate exchanger xCT in ventral hippocampus in resilience to stress. Neuron. 2017;96:402–13.e5.
Ferreira FR, Biojone C, Joca SR, Guimarães FS. Antidepressant-like effects of N-acetyl-L-cysteine in rats. Behavioural pharmacology. 2008;19:747–50.
Linck VM, Costa-Campos L, Pilz LK, Garcia CR, Elisabetsky E. AMPA glutamate receptors mediate the antidepressant-like effects of N-acetylcysteine in the mouse tail suspension test. Behav Pharmacol. 2012;23:171–7.
Chaudhry FA, Lehre KP, van Lookeren Campagne M, Ottersen OP, Danbolt NC, Storm-Mathisen J. Glutamate transporters in glial plasma membranes: highly differentiated localizations revealed by quantitative ultrastructural immunocytochemistry. Neuron. 1995;15:711–20.
Haugeto O, Ullensvang K, Levy LM, Chaudhry FA, Honoré T, Nielsen M, et al. Brain glutamate transporter proteins form homomultimers. J Biol Chem. 1996;271:27715–22.
Lehre KP, Danbolt NC. The number of glutamate transporter subtype molecules at glutamatergic synapses: chemical and stereological quantification in young adult rat brain. J Neurosci. 1998;18:8751–7.
Banasr M, Duman RS. Glial loss in the prefrontal cortex is sufficient to induce depressive-like behaviors. Biol psychiatry. 2008;64:863–70.
Gittins RA, Harrison PJ. A morphometric study of glia and neurons in the anterior cingulate cortex in mood disorder. J Affect Disord. 2011;133:328–32.
Codeluppi SA, Chatterjee D, Prevot TD, Bansal Y, Misquitta KA, Sibille E, et al. Chronic stress alters astrocyte morphology in mouse prefrontal cortex. Int J Neuropsychopharmacol. 2021;24:842–53.
Codeluppi SA, Xu M, Bansal Y, Lepack AE, Duric V, Chow M, et al. Prefrontal cortex astroglia modulate anhedonia-like behavior. Mol Psychiatry. 2023;28:4632–41.
Kofuji P, Araque A. G-Protein-Coupled receptors in astrocyte-neuron communication. Neuroscience. 2021;456:71–84.
Eriksson PS, Nilsson M, Wågberg M, Hansson E, Rönnbäck L. Kappa-opioid receptors on astrocytes stimulate L-type Ca2+ channels. Neuroscience. 1993;54:401–7.
Stiene-Martin A, Zhou R, Hauser KF. Regional, developmental, and cell cycle-dependent differences in mu, delta, and kappa-opioid receptor expression among cultured mouse astrocytes. Glia. 1998;22:249–59.
Xu M, Bruchas MR, Ippolito DL, Gendron L, Chavkin C. Sciatic nerve ligation-induced proliferation of spinal cord astrocytes is mediated by kappa opioid activation of p38 mitogen-activated protein kinase. J Neurosci. 2007;27:2570–81.
McLennan GP, Kiss A, Miyatake M, Belcheva MM, Chambers KT, Pozek JJ, et al. Kappa opioids promote the proliferation of astrocytes via Gbetagamma and beta-arrestin 2-dependent MAPK-mediated pathways. J Neurochem. 2008;107:1753–65.
Knoll AT, Carlezon WA Jr. Dynorphin, stress, and depression. Brain Res. 2010;1314:56–73.
Tejeda HA, Shippenberg TS, Henriksson R. The dynorphin/κ-opioid receptor system and its role in psychiatric disorders. Cell Mol Life Sci. 2012;69:857–96.
Zan GY, Sun X, Wang YJ, Liu R, Wang CY, Du WJ, et al. Amygdala dynorphin/κ opioid receptor system modulates depressive-like behavior in mice following chronic social defeat stress. Acta pharmacologica Sin. 2022;43:577–87.
Wang YJ, Zan GY, Xu C, Li XP, Shu X, Yao SY, et al. The claustrum-prelimbic cortex circuit through dynorphin/κ-opioid receptor signaling underlies depression-like behaviors associated with social stress etiology. Nat Commun. 2023;14:7903.
Bruchas MR, Schindler AG, Shankar H, Messinger DI, Miyatake M, Land BB, et al. Selective p38α MAPK deletion in serotonergic neurons produces stress resilience in models of depression and addiction. Neuron. 2011;71:498–511.
Zan GY, Wang YJ, Li XP, Fang JF, Yao SY, Du JY, et al. Amygdalar κ-opioid receptor-dependent upregulating glutamate transporter 1 mediates depressive-like behaviors of opioid abstinence. Cell Rep. 2021;37:109913.
Ehrich JM, Messinger DI, Knakal CR, Kuhar JR, Schattauer SS, Bruchas MR, et al. Kappa opioid receptor-induced aversion requires p38 MAPK activation in VTA dopamine neurons. J Neurosci. 2015;35:12917–31.
Bruchas MR, Land BB, Aita M, Xu M, Barot SK, Li S, et al. Stress-induced p38 mitogen-activated protein kinase activation mediates kappa-opioid-dependent dysphoria. J Neurosci. 2007;27:11614–23.
Knackstedt LA, LaRowe S, Mardikian P, Malcolm R, Upadhyaya H, Hedden S, et al. The role of cystine-glutamate exchange in nicotine dependence in rats and humans. Biol psychiatry. 2009;65:841–5.
Chen Y, Yao SY, Shu X, Wang YJ, Liu JG. Changes in mRNA and miRNA expression in the prelimbic cortex related to depression-like syndrome induced by chronic social defeat stress in mice. Behavioural brain Res. 2023;438:114211.
Zhu Y, Wang K, Ma T, Ji Y, Lou Y, Fu X, et al. Nucleus accumbens D1/D2 circuits control opioid withdrawal symptoms in mice. J Clin Invest. 2023;133:e163266.
Zhang J, Lu Y, Jia M, Bai Y, Sun L, Dong Z, et al. Kappa opioid receptor in nucleus accumbens regulates depressive-like behaviors following prolonged morphine withdrawal in mice. iScience. 2023;26:107536.
Belcheva MM, Clark AL, Haas PD, Serna JS, Hahn JW, Kiss A, et al. Mu and kappa opioid receptors activate ERK/MAPK via different protein kinase C isoforms and secondary messengers in astrocytes. J Biol Chem. 2005;280:27662–9.
Bruchas MR, Macey TA, Lowe JD, Chavkin C. Kappa opioid receptor activation of p38 MAPK is GRK3- and arrestin-dependent in neurons and astrocytes. J Biol Chem. 2006;281:18081–9.
Kofuji P, Araque A. Astrocytes and behavior. Annu Rev Neurosci. 2021;44:49–67.
Bridges R, Lutgen V, Lobner D, Baker DA. Thinking outside the cleft to understand synaptic activity: contribution of the cystine-glutamate antiporter (System xc-) to normal and pathological glutamatergic signaling. Pharmacol Rev. 2012;64:780–802.
Petr GT, Sun Y, Frederick NM, Zhou Y, Dhamne SC, Hameed MQ, et al. Conditional deletion of the glutamate transporter GLT-1 reveals that astrocytic GLT-1 protects against fatal epilepsy while neuronal GLT-1 contributes significantly to glutamate uptake into synaptosomes. J Neurosci. 2015;35:5187–201.
Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci. 2009;10:561–72.
John CS, Smith KL, Van’t Veer A, Gompf HS, Carlezon WA Jr, Cohen BM, et al. Blockade of astrocytic glutamate uptake in the prefrontal cortex induces anhedonia. Neuropsychopharmacology. 2012;37:2467–75.
McBean GJ. Cerebral cystine uptake: a tale of two transporters. Trends Pharmacol Sci. 2002;23:299–302.
Bentea E, Demuyser T, Van Liefferinge J, Albertini G, Deneyer L, Nys J, et al. Absence of system xc- in mice decreases anxiety and depressive-like behavior without affecting sensorimotor function or spatial vision. Prog Neuro-psychopharmacol Biol psychiatry. 2015;59:49–58.
Albertini G, Deneyer L, Ottestad-Hansen S, Zhou Y, Ates G, Walrave L, et al. Genetic deletion of xCT attenuates peripheral and central inflammation and mitigates LPS-induced sickness and depressive-like behavior in mice. Glia. 2018;66:1845–61.
Demuyser T, Deneyer L, Bentea E, Albertini G, Femenia T, Walrave L, et al. Slc7a11 (xCT) protein expression is not altered in the depressed brain and system xc- deficiency does not affect depression-associated behaviour in the corticosterone mouse model. World J Biol Psychiatry. 2019;20:381–92.
Chaki S. mGlu2/3 receptor antagonists as novel antidepressants. Trends Pharmacol Sci. 2017;38:569–80.
Kawasaki T, Ago Y, Yano K, Araki R, Washida Y, Onoe H, et al. Increased binding of cortical and hippocampal group II metabotropic glutamate receptors in isolation-reared mice. Neuropharmacology. 2011;60:397–404.
Nasca C, Bigio B, Zelli D, Nicoletti F, McEwen BS. Mind the gap: glucocorticoids modulate hippocampal glutamate tone underlying individual differences in stress susceptibility. Mol Psychiatry. 2015;20:755–63.
Feyissa AM, Woolverton WL, Miguel-Hidalgo JJ, Wang Z, Kyle PB, Hasler G, et al. Elevated level of metabotropic glutamate receptor 2/3 in the prefrontal cortex in major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:279–83.
Muguruza C, Miranda-Azpiazu P, Díez-Alarcia R, Morentin B, González-Maeso J, Callado LF, et al. Evaluation of 5-HT2A and mGlu2/3 receptors in postmortem prefrontal cortex of subjects with major depressive disorder: effect of antidepressant treatment. Neuropharmacology. 2014;86:311–8.
McOmish CE, Pavey G, Gibbons A, Hopper S, Udawela M, Scarr E, et al. Lower [3H]LY341495 binding to mGlu2/3 receptors in the anterior cingulate of subjects with major depressive disorder but not bipolar disorder or schizophrenia. J Affect Disord. 2016;190:241–8.
Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta Neuropathol. 2010;119:7–35.
Siracusa R, Fusco R, Cuzzocrea S. Astrocytes: role and functions in brain pathologies. Front Pharmacol. 2019;10:1114.
Ongür D, Drevets WC, Price JL. Glial reduction in the subgenual prefrontal cortex in mood disorders. Proc Natl Acad Sci USA. 1998;95:13290–5.
McLaughlin JP, Li S, Valdez J, Chavkin TA, Chavkin C. Social defeat stress-induced behavioral responses are mediated by the endogenous kappa opioid system. Neuropsychopharmacology. 2006;31:1241–8.
Donahue RJ, Landino SM, Golden SA, Carroll FI, Russo SJ, Carlezon WA Jr. Effects of acute and chronic social defeat stress are differentially mediated by the dynorphin/kappa-opioid receptor system. Behav Pharmacol. 2015;26:654–63.
Land BB, Bruchas MR, Schattauer S, Giardino WJ, Aita M, Messinger D, et al. Activation of the kappa opioid receptor in the dorsal raphe nucleus mediates the aversive effects of stress and reinstates drug seeking. Proc Natl Acad Sci USA. 2009;106:19168–73.
Galeotti N, Ghelardini C. Selective modulation of the PKCɛ/p38MAP kinase signalling pathway for the antidepressant-like activity of amitriptyline. Neuropharmacology. 2012;62:289–96.
Grassmé H, Jernigan PL, Hoehn RS, Wilker B, Soddemann M, Edwards MJ, et al. Inhibition of acid sphingomyelinase by antidepressants counteracts stress-induced activation of P38-Kinase in major depression. Neurosignals. 2015;23:84–92.
Bruchas MR, Chavkin C. Kinase cascades and ligand-directed signaling at the kappa opioid receptor. Psychopharmacology. 2010;210:137–47.
Liu-Chen LY, Huang P. Signaling underlying kappa opioid receptor-mediated behaviors in rodents. Front Neurosci. 2022;16:964724.
Wadenberg ML. A review of the properties of spiradoline: a potent and selective kappa-opioid receptor agonist. CNS Drug Rev. 2003;9:187–98.
Mague SD, Pliakas AM, Todtenkopf MS, Tomasiewicz HC, Zhang Y, Stevens WC Jr., et al. Antidepressant-like effects of kappa-opioid receptor antagonists in the forced swim test in rats. J Pharmacol Exp Ther. 2003;305:323–30.
Dalefield ML, Scouller B, Bibi R, Kivell BM. The kappa opioid receptor: a promising therapeutic target for multiple pathologies. Front Pharmacol. 2022;13:837671.
Margolis EB, Wallace TL, Van Orden LJ, Martin WJ. Differential effects of novel kappa opioid receptor antagonists on dopamine neurons using acute brain slice electrophysiology. PLoS One. 2020;15:e0232864.
Van’t Veer A, Carlezon WA Jr. Role of kappa-opioid receptors in stress and anxiety-related behavior. Psychopharmacology. 2013;229:435–52.
Azocar VH, Sepúlveda G, Ruiz C, Aguilera C, Andrés ME, Fuentealba JA. The blocking of kappa-opioid receptor reverses the changes in dorsolateral striatum dopamine dynamics during the amphetamine sensitization. J Neurochem. 2019;148:348–58.
Wong S, Le GH, Vasudeva S, Teopiz KM, Phan L, Meshkat S, et al. Preclinical and clinical efficacy of kappa opioid receptor antagonists for depression: a systematic review. J Affect Disord. 2024;362:816–27.
Smirnova L, Gräfe A, Seiler A, Schumacher S, Nitsch R, Wulczyn FG. Regulation of miRNA expression during neural cell specification. Eur J Neurosci. 2005;21:1469–77.
Mor E, Cabilly Y, Goldshmit Y, Zalts H, Modai S, Edry L, et al. Species-specific microRNA roles elucidated following astrocyte activation. Nucleic Acids Res. 2011;39:3710–23.
Schratt GM, Tuebing F, Nigh EA, Kane CG, Sabatini ME, Kiebler M, et al. A brain-specific microRNA regulates dendritic spine development. Nature. 2006;439:283–9.
Schratt G. microRNAs at the synapse. Nat Rev Neurosci. 2009;10:842–9.
Baudry A, Mouillet-Richard S, Schneider B, Launay JM, Kellermann O. miR-16 targets the serotonin transporter: a new facet for adaptive responses to antidepressants. Science. 2010;329:1537–41.
Issler O, Haramati S, Paul ED, Maeno H, Navon I, Zwang R, et al. MicroRNA 135 is essential for chronic stress resiliency, antidepressant efficacy, and intact serotonergic activity. Neuron. 2014;83:344–60.
Issler O, Chen A. Determining the role of microRNAs in psychiatric disorders. Nat Rev Neurosci. 2015;16:201–12.
Torres-Berrío A, Nouel D, Cuesta S, Parise EM, Restrepo-Lozano JM, Larochelle P, et al. MiR-218: a molecular switch and potential biomarker of susceptibility to stress. Mol Psychiatry. 2020;25:951–64.
McIntyre RS, Jain R. Glutamatergic modulators for major depression from theory to clinical use. CNS Drugs. 2024;38:869–90.
Wang YJ, Rasakham K, Huang P, Chudnovskaya D, Cowan A, Liu-Chen LY. Sex difference in κ-opioid receptor (KOPR)-mediated behaviors, brain region KOPR level and KOPR-mediated guanosine 5’-O-(3-[35S]thiotriphosphate) binding in the guinea pig. J pharmacology Exp therapeutics. 2011;339:438–50.
Russell SE, Rachlin AB, Smith KL, Muschamp J, Berry L, Zhao Z, et al. Sex differences in sensitivity to the depressive-like effects of the kappa opioid receptor agonist U-50488 in rats. Biol psychiatry. 2014;76:213–22.
Chen Y, Jiang Y, Jiang X, Zhai C, Wang Y, Xu C. Identification and experimental validation of hub genes underlying depressive-like behaviors induced by chronic social defeat stress. Front Pharmacol. 2024;15:1472468.
Golden SA, Covington HE 3rd, Berton O, Russo SJ. A standardized protocol for repeated social defeat stress in mice. Nat Protoc. 2011;6:1183–91.
Chen Y, Wang CY, Zan GY, Yao SY, Deng YZ, Shu XL, et al. Upregulation of dynorphin/kappa opioid receptor system in the dorsal hippocampus contributes to morphine withdrawal-induced place aversion. Acta pharmacologica Sin. 2023;44:538–45.
Christianson JP, Flyer-Adams JG, Drugan RC, Amat J, Daut RA, Foilb AR, et al. Learned stressor resistance requires extracellular signal-regulated kinase in the prefrontal cortex. Front Behav Neurosci. 2014;8:348.
Ameneiro L, Zalcman G, Robles A, Romano A. Characteristics of the Reminder that Triggers Object Recognition Memory Reconsolidation in Mice. Neuroscience. 2022;497:206–14.
Griffin WC 3rd, Haun HL, Hazelbaker CL, Ramachandra VS, Becker HC. Increased extracellular glutamate in the nucleus accumbens promotes excessive ethanol drinking in ethanol dependent mice. Neuropsychopharmacology. 2014;39:707–17.
Acknowledgements
This research was supported by the Major Project of the Science and Technology Innovation 2030 of China (STI2030-Major Projects 2021ZD0202900 to J.-G.L., 2021ZD0203500 to Y.-J.W.), from the National Natural Science Foundation of China (82030112 to J.-G.L., 82273904 to G.-Y.Z.), from the Key R&D Program of Shandong Province, China (2024CXPT029 to Y.J. W), from the Fundamental Research Projects of Science & Technology Innovation and development Plan in Yantai City (No.2024JCYJ045 to Y.J. W), from Science and Technology Commission of Shanghai Municipality 23ZR1474900 to Y.-J.W.), and from the Taishan Scholars Program and Shandong Laboratory Program (SYS202205).
Author information
Authors and Affiliations
Contributions
Yu-Jun Wang, Jing-Gen Liu, Jiaoqiao Fang and Xiaomei Shao designed the experiments. Yexiang Chen, Liu-Bin Guo and Guiying Zan performed the experiments and statistical analysis with the assistance of Chi Xu, Song-Yu Yao, Shuo Wu, Xingcong Jiang, Zihan Liu, Jian-Dong Long, Boyu Liu and Xiaofen He. The manuscript was written by Yexiang Chen, Jing-Gen Liu and Yu-Jun Wang, and was revised by Xiaomei Shao and Jiaoqiao Fang.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
All animal experiments and procedures were approved by the Animal Care and Use Committee of Shanghai Institute of Materia Medica, Chinese Academy of Sciences (Approval No. 2022-07-LJG-76).Written informed consent was not required, as no human participant was involved in this study. All methods were performed in accordance with the relevant guidelines and regulations.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, Y., Guo, LB., Zan, GY. et al. Astroglial kappa opioid receptor-mediated reduction of glutamate exchanger xCT in the prelimbic cortex underlies chronic stress-induced depressive-like behaviors. Mol Psychiatry (2025). https://doi.org/10.1038/s41380-025-03441-y
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41380-025-03441-y