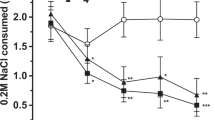
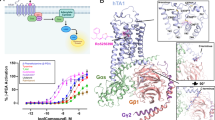
Abstract
Trace amine-associated receptor 1 (TAAR1) is a novel target for antipsychotic and potentially mood-stabilizing and anti-addictive drugs, offering a new mechanism by modulating dopaminergic, serotonergic, and glutamatergic neurotransmission. TAAR1 agonists from a prior amino oxazolines series were studied preclinically and clinically, but development of partial agonist RO5263397 was halted due to poor metabolization by N-glucuronidation in individuals with UGT2B10 splice site mutations. A medicinal chemistry program subsequently identified new potent and selective TAAR1 ligands from the morpholine series. Two selective partial agonists, RO6799477 and RO6889450, were advanced and showed antipsychotic, stress-response-modulating, and anti-addictive-like activity in rodent models. They reduced PCP- and cocaine-induced hyperlocomotion, potentiated olanzapine’s effect, partially reversed cocaine-induced facilitation of intracranial self-stimulation and demonstrated anxiolytic-like properties in the stress-induced hyperthermia test. Neural activation profiles, measured by pharmacological MRI, differed from first and second-generation antipsychotics. Non-clinical drug abuse liability studies with RO6889450 indicated a favorable low abuse liability profile and suggested a lack of reinforcing effects. Preclinical safety studies indicated a favorable profile with clinically monitorable and reversible findings. Both compounds progressed into Phase I single and multiple ascending dose studies in healthy volunteers. RO6799477 was well tolerated, with rapid absorption and dose-proportional pharmacokinetics, though higher doses led to nervous system and cardiovascular adverse events. RO6889450 was well tolerated up to 300 mg; at 450 mg, dose-limiting adverse events included postural tachycardia and erythema. Together, these results indicate the translational potential and support further clinical exploration of TAAR1 agonists as a novel therapeutic option for neuropsychiatric disorders, particularly schizophrenia and substance use disorder.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
Information regarding the data and materials for this study is provided below: The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request. Relevant data supporting the findings, including pharmacological profiles and pharmacokinetic parameters, are included within this article and its supplementary information files. Clinical trial data are registered and accessible via ClinicalTrials.gov under the identifiers NCT01893437, NCT02164266, and NCT02699372.
References
Lindemann L, Hoener MC. A renaissance in trace amines inspired by a novel GPCR family. Trends Pharmacol Sci. 2005;26:274–81.
Lindemann L, Meyer CA, Jeanneau K, Bradaia A, Ozmen L, Bluethmann H, et al. Trace amine-associated receptor 1 modulates dopaminergic activity. J Pharmacol Exp Ther. 2008;324:948–56.
Borowsky B, Adham N, Jones KA, Raddatz R, Artymyshyn R, Ogozalek KL, et al. Trace amines: identification of a family of mammalian G protein-coupled receptors. Proc Natl Acad Sci USA. 2001;98:8966–71.
Raab S, Wang HY, Uhles S, Cole N, Alvarez-Sanchez R, Künnecke B, et al. Incretin-like effects of small molecule trace amine-associated receptor 1 agonists. Mol Metab. 2016;5:47–56.
Revel FG, Moreau JL, Pouzet B, Mory R, Bradaia A, Buchy D, et al. A new perspective for schizophrenia: TAAR1 agonists reveal antipsychotic- and antidepressant-like activity, improve cognition and control body weight. Mol Psychiatry. 2013;18:543–56.
Ito J, Ito M, Nambu H, Fujikawa T, Tanaka K, Iwaasa H, et al. Anatomical and histological profiling of orphan G-protein-coupled receptor expression in gastrointestinal tract of C57BL/6J mice. Cell Tissue Res. 2009;338:257–69.
Revel FG, Meyer CA, Bradaia A, Jeanneau K, Calcagno E, Andre CB, et al. Brain-specific overexpression of trace amine-associated receptor 1 alters monoaminergic neurotransmission and decreases sensitivity to amphetamine. Neuropsychopharmacology. 2012;37:2580–92.
Revel FG, Moreau JL, Gainetdinov RR, Bradaia A, Sotnikova TD, Mory R, et al. TAAR1 activation modulates monoaminergic neurotransmission, preventing hyperdopaminergic and hypoglutamatergic activity. Proc Natl Acad Sci USA. 2011;108:8485–90.
Dedic N, Jones PG, Hopkins SC, Lew R, Shao L, Campbell JE, et al. SEP-363856, a Novel Psychotropic Agent with a Unique, Non-D(2) Receptor Mechanism of Action. J Pharmacol Exp Ther. 2019;371:1–14.
Liu JF, Li JX. TAAR1 in addiction: looking beyond the tip of the iceberg. Front Pharmacol. 2018;9:279.
Wu R, Liu J, Li JX. Trace amine-associated receptor 1 and drug abuse. Adv Pharmacol. 2022;93:373–401.
Pei Y, Mortas P, Hoener MC, Canales JJ. Selective activation of the trace amine-associated receptor 1 decreases cocaine’s reinforcing efficacy and prevents cocaine-induced changes in brain reward thresholds. Prog Neuropsychopharmacol Biol Psychiatry. 2015;63:70–75.
Asif-Malik A, Hoener MC, Canales JJ. Interaction between the trace amine-associated receptor 1 and the dopamine D(2) receptor controls cocaine’s neurochemical actions. Sci Rep. 2017;7:13901.
Harmeier A, Obermueller S, Meyer CA, Revel FG, Buchy D, Chaboz S, et al. Trace amine-associated receptor 1 activation silences GSK3beta signaling of TAAR1 and D2R heteromers. Eur Neuropsychopharmacol. 2015;25:2049–61.
Liu J, Wu R, Li JX. TAAR1 and psychostimulant addiction. Cell Mol Neurobiol. 2020;40:229–38.
Berry MD, Gainetdinov RR, Hoener MC, Shahid M. Pharmacology of human trace amine-associated receptors: Therapeutic opportunities and challenges. Pharmacol Ther. 2017;180:161–80.
Schwartz MD, Canales JJ, Zucchi R, Espinoza S, Sukhanov I, Gainetdinov RR. Trace amine-associated receptor 1: a multimodal therapeutic target for neuropsychiatric diseases. Expert Opin Ther Targets. 2018;22:513–26.
Howes OD, Kambeitz J, Kim E, Stahl D, Slifstein M, Abi-Dargham A, et al. The nature of dopamine dysfunction in schizophrenia and what this means for treatment. Arch Gen Psychiatry. 2012;69:776–86.
Poels EMP, Kegeles LS, Kantrowitz JT, Javitt DC, Lieberman JA, Abi-Darghama A, et al. Glutamatergic abnormalities in schizophrenia: A review of proton MRS findings. Schizophr Res. 2014;152:325–32.
Belmaker RH, Agam G. Mechanisms of disease: major depressive disorder. New Engl J Med. 2008;358:55–68.
Racagni G, Popoli M. Cellular and molecular mechanisms in the long-term action of antidepressants. Dialogues Clin Neurosci. 2008;10:385–400.
Sen S, Sanacora G. Major depression: emerging therapeutics. Mt Sinai J Med. 2008;75:204–25.
Juckel G, Schlagenhauf F, Koslowski M, Filonov D, Wustenberg T, Villringer A, et al. Dysfunction of ventral striatal reward prediction in schizophrenic patients treated with typical, not atypical, neuroleptics. Psychopharmacology (Berl). 2006;187:222–8.
Emsley R, Chiliza B, Asmal L, Harvey BH. The nature of relapse in schizophrenia. BMC Psychiatry. 2013;13:50.
Mas S, Gassó P, de Bobadilla RF, Arnaiz JA, Bernardo M, Lafuente A. Secondary nonmotor negative symptoms in healthy volunteers after single doses of haloperidol and risperidone: a double-blind, crossover, placebo-controlled trial. Hum Psychopharm Clin. 2013;28:586–93.
Dorph-Petersen KA, Pierri JN, Perel JM, Sun ZX, Sampson AR, Lewis DA. The influence of chronic exposure to antipsychotic medications on brain size before and after tissue fixation: A comparison of haloperidol and olanzapine in macaque monkeys. Neuropsychopharmacology. 2005;30:1649–61.
Ho BC, Andreasen NC, Ziebell S, Pierson R, Magnotta V. Long-term antipsychotic treatment and brain volumes. Arch Gen Psychiat. 2011;68:128–37.
Revel FG, Moreau JL, Gainetdinov RR, Ferragud A, Velazquez-Sanchez C, Sotnikova TD, et al. Trace amine-associated receptor 1 partial agonism reveals novel paradigm for neuropsychiatric therapeutics. Biol Psychiatry. 2012;72:934–42.
Dedic N, Wang L, Hajos-Korcsok E, Hecksher-Sorensen J, Roostalu U, Vickers SP, et al. TAAR1 agonists improve glycemic control, reduce body weight and modulate neurocircuits governing energy balance and feeding. Mol Metab. 2024;80:101883.
Koblan KS, Kent J, Hopkins SC, Krystal JH, Cheng H, Goldman R, et al. A non-D2-receptor-binding drug for the treatment of schizophrenia. N Engl J Med. 2020;382:1497–506.
Dedic N, Dworak H, Zeni C, Rutigliano G, Howes OD. Therapeutic potential of TAAR1 agonists in schizophrenia: evidence from preclinical models and clinical studies. Int J Mol Sci. 2021;22:13185.
Correll CU, Koblan KS, Hopkins SC, Li Y, Heather D, Goldman R, et al. Safety and effectiveness of ulotaront (SEP-363856) in schizophrenia: results of a 6-month, open-label extension study. NPJ Schizophr. 2021;7:63.
Agren R, Betari N, Saarinen M, Zeberg H, Svenningsson P, Sahlholm K. In vitro comparison of ulotaront (SEP-363856) and ralmitaront (RO6889450): two TAAR1 agonist candidate antipsychotics. Int J Neuropsychopharmacol. 2023;26:599–606.
Trussardi R, Iding H. Process for the preparation of chiral 2-aryl morpholines. WO2015086495A1 edn2015.
Galley G, Hoener M, Norcross R, Pflieger P. 5-Ethyl-4-methyl-pyrazole-3-carboxamide derivative having activity as agonist of TAAR1. WO2017157873A1 edn2017.
Rodefer JS, Nguyen TN, Karlsson JJ, Arnt J. Reversal of subchronic PCP-induced deficits in attentional set shifting in rats by sertindole and a 5-HT6 receptor antagonist: comparison among antipsychotics. Neuropsychopharmacology. 2008;33:2657–66.
Bruns A, Künnecke B, Risterucci C, Moreau JL, von Kienlin M. Validation of cerebral blood perfusion imaging as a modality for quantitative pharmacological MRI in Rats. Magn Reson Med. 2009;61:1451–8.
Bruns A, Mueggler T, Künnecke B, Risterucci C, Prinssen EP, Wettstein JG, et al. Domain gauges: A reference system for multivariate profiling of brain fMRI activation patterns induced by psychoactive drugs in rats. Neuroimage. 2015;112:70–85.
Bradaia A, Trube G, Stalder H, Norcross RD, Ozmen L, Wettstein JG, et al. The selective antagonist EPPTB reveals TAAR1-mediated regulatory mechanisms in dopaminergic neurons of the mesolimbic system. Proc Natl Acad Sci USA. 2009;106:20081–6.
Fowler S, Kletzl H, Finel M, Manevski N, Schmid P, Tuerck D, et al. A UGT2B10 splicing polymorphism common in african populations may greatly increase drug exposure. J Pharmacol Exp Ther. 2015;352:358–67.
Gainetdinov RR, Hoener MC, Berry MD. Trace amines and their receptors. Pharmacol Rev. 2018;70:549–620.
Zhang S, Li B, Lovatt D, Xu J, Song D, Goldman SA, et al. 5-HT2B receptors are expressed on astrocytes from brain and in culture and are a chronic target for all five conventional serotonin-specific reuptake inhibitors. Neuron Glia Biol. 2010;6:113–25.
Synan C, Bowen C, Heal DJ, Froger-Colleaux C, Beardsley PM, Dedic N, et al. Ulotaront, a novel TAAR1 agonist with 5-HT1A agonist activity, lacks abuse liability and attenuates cocaine cue-induced relapse in rats. Drug Alcohol Depend. 2022;231:109261.
Acknowledgements
We acknowledge the continuous support of F. Hoffmann-La Roche Ltd.
Funding
The work was funded by F. Hoffmann-La Roche Ltd.
Author information
Authors and Affiliations
Contributions
CF contributed to the analysis and interpretation of data and drafted and revised the manuscript. DB contributed to the acquisition, analysis and interpretation of data and revised the manuscript. SM contributed to the conception and design of the work, the acquisition, analysis, and interpretation of data, and drafted and revised the manuscript. AP contributed to the conception and design of the work, the acquisition, analysis, and interpretation of data, and drafted and revised the manuscript. BJ contributed to the acquisition, analysis, and interpretation of data, and revised the manuscript. RN contributed to the conception and design of the work, the acquisition, analysis, and interpretation of data, and revised the manuscript. PP contributed to the acquisition, analysis, and interpretation of data. SS contributed to the analysis and interpretation of data. AGW contributed to the conception and design of the work, the acquisition, analysis, and interpretation of data, and drafted and revised the manuscript. BK contributed to the acquisition, analysis, and interpretation of data, and revised the manuscript. CS contributed to the acquisition, analysis, and interpretation of data, and revised the manuscript. MMo contributed to the acquisition, analysis, and interpretation of data, and revised the manuscript. MMa contributed to the acquisition, analysis, and interpretation of data, and revised the manuscript. SH revised the manuscript. IG revised the manuscript. MCH contributed to the conception and design of the work, the acquisition, analysis, and interpretation of data, and drafted and revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
CF, DB, SM, AP, BJ, RN, PP, SS, AGW, BK, CS, MM, MM, SH, IG and MCH are employees of F. Hoffmann-La Roche, Ltd. and may own stock or stock options.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fournier, C., Buchy, D., Mohr, S. et al. Novel selective morpholine trace amine-associated receptor 1 partial agonists show promising preclinical effects for neuropsychiatric disorders and are well tolerated in healthy volunteers. Mol Psychiatry (2026). https://doi.org/10.1038/s41380-026-03465-y
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41380-026-03465-y