Abstract
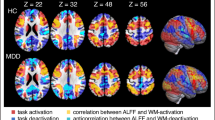
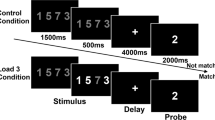
Major depressive disorder (MDD) is often accompanied by severe impairments in working memory (WM). Neuroimaging studies investigating the mechanisms underlying these impairments have produced conflicting results. It remains unclear whether MDD patients show hyper- or hypoactivity in WM-related brain regions and how potential aberrations in WM processing may contribute to the characteristic dysregulation of cognition–emotion interactions implicated in the maintenance of the disorder. In order to shed light on these questions and to overcome limitations of previous studies, we applied a multivoxel pattern classification approach to investigate brain activity in large samples of MDD patients (N = 57) and matched healthy controls (N = 61) during a WM task that incorporated positive, negative, and neutral stimuli. Results showed that patients can be distinguished from healthy controls with good classification accuracy based on functional activation patterns. ROI analyses based on the classification weight maps showed that during WM, patients had higher activity in the left DLPFC and the dorsal ACC. Furthermore, regions of the default-mode network (DMN) were less deactivated in patients. As no performance differences were observed, we conclude that patients required more effort, indexed by more activity in WM-related regions, to successfully perform the task. This increased effort might be related to difficulties in suppressing task-irrelevant information reflected by reduced deactivation of regions within the DMN. Effects were most pronounced for negative and neutral stimuli, thus pointing toward important implications of aberrations in WM processes in cognition–emotion interactions in MDD.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Millan MJ, Agid Y, Brüne M, Bullmore ET, Carter CS, Clayton NS, et al. Cognitive dysfunction in psychiatric disorders: characteristics, causes and the quest for improved therapy. Nat Rev Drug Discov. 2012;11:141–68.
Wager TD, Smith EE. Neuroimaging studies of working memory: a meta-analysis. Cogn Affect Behav Neurosci. 2003;3:255–74.
Glahn DC, Curran JE, Winkler AM, Carless MA, Kent JW, Charlesworth JC, et al. High dimensional endophenotype ranking in the search for major depression risk genes. Biol Psychiatry. 2012;71:6–14.
Duncan J, Owen AM. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci. 2000;23:475–83.
Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: an update. Trends Cogn Sci. 2004;8:539–46.
Fitzgerald PB, Srithiran A, Benitez J, Daskalakis ZZ, Oxley TJ, Kulkarni J, et al. An fMRI study of prefrontal brain activation during multiple tasks in patients with major depressive disorder. Human Brain Mapp. 2008;29:490–501.
Harvey P-O, Fossati P, Pochon J-B, Levy R, LeBastard G, Lehéricy S, et al. Cognitive control and brain resources in major depression: an fMRI study using the n-back task. Neuroimage. 2005;26:860–9.
Matsuo K,Glahn D,A M Peluso M,Hatch JP,Monkul ES,Najt P, et al. Prefrontal hyperactivation during working memory task in untreated individuals with major depressive disorder. Mol Psychiatry. 2007;12:158–66.
Grimm S, Boesiger P, Beck J, Schuepbach D, Bermpohl F, Walter M, et al. Altered negative BOLD responses in the default-mode network during emotion processing in depressed subjects. Neuropsychopharmacolgy. 2009;34:932–43.
Pu S, Yamada T, Yokoyama K, Matsumura H, Kobayashi H, Sasaki N, et al. A multi-channel near-infrared spectroscopy study of prefrontal cortex activation during working memory task in major depressive disorder. Neurosci Res. 2011;70:91–7.
Hugdahl K, Rund BR, Lund A, Asbjørnsen A, Egeland J, Ersland L, et al. Brain activation measured with fMRI during a mental arithmetic task in schizophrenia and major depression. Am J Psychiatry. 2004;161:286–93.
Wang XL, Du MY, Chen TL, Chen ZQ, Huang XQ, Luo Y, et al. Neural correlates during working memory processing in major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2015;56:101–8.
Müller VI, Cieslik EC, Serbanescu I, Laird AR, Fox PT, Eickhoff SB. Altered brain activity in unipolar depression revisited: meta-analyses of neuroimaging studies. JAMA Psychiatry. 2017;74:47–55.
Aoki R, Sato H, Katura T, Utsugi K, Koizumi H, Matsuda R, et al. Relationship of negative mood with prefrontal cortex activity during working memory tasks: an optical topography study. Neurosci Res. 2011;70:189–96.
Gray JR, Braver TS, Raichle ME. Integration of emotion and cognition in the lateral prefrontal cortex. Proc Natl Acad Sci USA. 2002;99:4115–20.
Siemer M. Mood-congruent cognitions constitute mood experience. Emotion. 2005;5:296–308.
Mathews A, MacLeod C. Cognitive vulnerability to emotional disorders. Annu Rev Clin Psychol. 2005;1:167–95.
Rusting CL. Personality, mood, and cognitive processing of emotional information: three conceptual frameworks. Psychol Bull. 1998;124:165–96.
Gotlib IH, Krasnoperova E, Yue DN, Joormann J. Attentional biases for negative interpersonal stimuli in clinical depression. J Abnorm Psychol. 2004;113:121–35.
Joormann J, Tanovic E. Cognitive vulnerability to depression: examining cognitive control and emotion regulation. Curr Opin Psychol. 2015;4:86–92.
Hamilton JP, Furman DJ, Chang C, Thomason ME, Dennis E, Gotlib IH. Default-mode and task-positive network activity in major depressive disorder: implications for adaptive and maladaptive rumination. Biol Psychiatry. 2011;70:327–33.
Rose EJ, Simonotto E, Ebmeier KP. Limbic over-activity in depression during preserved performance on the n-back task. Neuroimage. 2006;29:203–15.
Võ MLH, Conrad M, Kuchinke L, Urton K, Hofmann MJ, Jacobs AM. The Berlin Affective Word List Reloaded (BAWL-R). Behav Res Methods. 2009;41:534–8.
Grimm S, Weigand A, Kazzer P, Jacobs AM, Bajbouj M. Neural mechanisms underlying the integration of emotion and working memory. Neuroimage. 2012;61:1188–94.
Scheidegger M, Henning A, Walter M, Boeker H, Weigand A, Seifritz E, et al. Effects of ketamine on cognition–emotion interaction in the brain. Neuroimage. 2016;124:8–15.
Beck AT, Steer RA, Brown GK. BDI-II, Beck depression inventory: manual. San Antonio, TX, Boston: Psychological Corp., Harcourt Brace; 1996.
Treynor W, Gonzalez R, Nolen-Hoeksema S. Rumination reconsidered: a psychometric analysis. Cogn Ther Res. 2003;27:247–59.
Patel MJ, Khalaf A, Aizenstein HJ. Studying depression using imaging and machine learning methods. NeuroImage Clin. 2016;10:115–23.
Guyon I, Weston J, Barnhill S, Vapnik V. Gene selection for cancer classification using support vector machines. Mach Learn. 2002;46:389–422.
Chen Y-W, Lin C-J. Combining SVMs with various feature selection strategies. Feature extraction.. Berlin, Heidelberg: Springer; 2006. p. 315–24.
Ojala M, Garriga GC. Permutation tests for studying classifier performance. J Mach Learn Res. 2010;11:1833–63.
Broyd SJ, Demanuele C, Debener S, Helps SK, James CJ, Sonuga-Barke EJS. Default-mode brain dysfunction in mental disorders: a systematic review. Neurosci Biobehav Rev. 2009;33:279–96.
Kambeitz J, Cabral C, Sacchet MD, Gotlib IH, Zahn R, Serpa MH, et al. Detecting neuroimaging biomarkers for depression: a meta-analysis of multivariate pattern recognition studies. Biol Psychiatry. 2017;82:330–8.
Dolcos F, McCarthy G. Brain systems mediating cognitive interference by emotional distraction. J Neurosci. 2006;26:2072–9.
Phillips ML,Ladouceur CD,Drevets WC, A neural model of voluntary and automatic emotion regulation: implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Mol Psychiatry. 2008;13:829–57.
Gilboa-Schechtman E, Ben-Artzi E, Jeczemien P, Marom S, Hermesh H. Depression impairs the ability to ignore the emotional aspects of facial expressions: evidence from the Garner task. Cogn Emot. 2004;18:209–31.
Disner SG, Beevers CG, Haigh EAP, Beck AT. Neural mechanisms of the cognitive model of depression. Nat Rev Neurosci. 2011;12:467–77.
Fox MD, Snyder AZ, Vincent JL, Corbetta M, Essen DCV, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA. 2005;102:9673–8.
Leech R, Kamourieh S, Beckmann CF, Sharp DJ. Fractionating the default mode network: distinct contributions of the ventral and dorsal posterior cingulate cortex to cognitive control. J Neurosci. 2011;31:3217–24.
Bonnelle V, Leech R, Kinnunen KM, Ham TE, Beckmann CF, Boissezon XD, et al. Default mode network connectivity predicts sustained attention deficits after traumatic brain injury. J Neurosci. 2011;31:13442–51.
Weissman DH, Roberts KC, Visscher KM, Woldorff MG. The neural bases of momentary lapses in attention. Nat Neurosci. 2006;9:971–8.
Grimm S, Ernst J, Boesiger P, Schuepbach D, Boeker H, Northoff G. Reduced negative BOLD responses in the default-mode network and increased self-focus in depression. World J Biol Psychiatry. 2011;12:627–37.
Diener C, Kuehner C, Brusniak W, Ubl B, Wessa M, Flor H. A meta-analysis of neurofunctional imaging studies of emotion and cognition in major depression. Neuroimage. 2012;61:677–85.
Pizzagalli DA. Frontocingulate dysfunction in depression: toward biomarkers of treatment response. Neuropsychopharmacology. 2011;36:183–206.
Braga RM, Sharp DJ, Leeson C, Wise RJS, Leech R. Echoes of the brain within default mode, association, and heteromodal cortices. J Neurosci. 2013;33:14031–9.
Philip NS, Sweet LH, Tyrka AR, Carpenter SL, Albright SE, Price LH, et al. Exposure to childhood trauma is associated with altered n-back activation and performance in healthy adults: implications for a commonly used working memory task. Brain Imaging Behav. 2016;10:124–35.
Nolan SA, Roberts JE, Gotlib IH. Neuroticism and ruminative response style as predictors of change in depressive symptomatology. Cogn Ther Res. 1998;22:445–55.
Craig AD. Emotional moments across time: a possible neural basis for time perception in the anterior insula. Philos Trans R Soc Lond B Biol Sci. 2009;364:1933–42.
Gu X, Hof PR, Friston KJ, Fan J. Anterior insular cortex and emotional awareness. J Comp Neurol. 2013;521:3371–88.
Dosenbach NUF, Fair DA, Cohen AL, Schlaggar BL, Petersen SE. A dual-networks architecture of top-down control. Trends Cogn Sci. 2008;12:99–105.
Delaveau P, Jabourian M, Lemogne C, Guionnet S, Bergouignan L, Fossati P. Brain effects of antidepressants in major depression: a meta-analysis of emotional processing studies. J Affect Disord. 2011;130:66–74.
Döhnel K, Sommer M, Ibach B, Rothmayr C, Meinhardt J, Hajak G. Neural correlates of emotional working memory in patients with mild cognitive impairment. Neuropsychologia. 2008;46:37–48.
LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–84.
Lau MA, Christensen BK, Hawley LL, Gemar MS, Segal ZV. Inhibitory deficits for negative information in persons with major depressive disorder. Psychol Med. 2007;37:1249–59.
Acknowledgements
We thank the European Commission (grant number H2020-634541) and the German Research Foundation (grant number GR 4510/2-1) for funding this research. Furthermore, we thank our participants for giving time to take part in this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
KB is co-founder and co-owner of Computomics GmbH. The remaining authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Gärtner, M., Ghisu, M.E., Scheidegger, M. et al. Aberrant working memory processing in major depression: evidence from multivoxel pattern classification. Neuropsychopharmacol 43, 1972–1979 (2018). https://doi.org/10.1038/s41386-018-0081-1
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41386-018-0081-1
This article is cited by
-
Aberrant associations between neuronal resting-state fluctuations and working memory-induced activity in major depressive disorder
Molecular Psychiatry (2025)
-
Altered patterns of central executive, default mode and salience network activity and connectivity are associated with current and future depression risk in two independent young adult samples
Molecular Psychiatry (2023)
-
Prefrontal cortex and depression
Neuropsychopharmacology (2022)
-
Neural function during emotion regulation and future depressive symptoms in youth at risk for affective disorders
Neuropsychopharmacology (2021)
-
Predicting Response to Brain Stimulation in Depression: a Roadmap for Biomarker Discovery
Current Behavioral Neuroscience Reports (2021)