Abstract
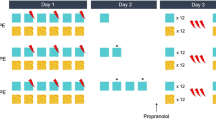
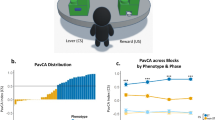
When memories are retrieved they become labile, and subject to alteration by a process known as reconsolidation. Disruption of memory reconsolidation decreases the performance of learned responses, which is often attributed to erasure of the memory; in the case of Pavlovian learning, to a loss of the association between a conditioned stimulus (CS) and unconditioned stimulus (US). However, an alternative interpretation is that disrupting reconsolidation does not erase memories, but blunts their emotional/motivational impact. It is difficult to parse the predictive vs. emotional/motivational value of CSs in non-human animals, but studies on variation in the form of conditioned responses (CRs) in a Pavlovian conditioned approach task suggest a way to do this. In this task a lever-CS paired with a food reward (US) acquires predictive value in all rats, but is attributed with emotional/motivational value to a greater extent in some rats (sign-trackers) than others (goal-trackers). We report that the post-retrieval administration of propranolol selectively attenuates a sign-tracking CR, and the associated neural activation of brain “motive circuits”, while having no effect on conditioned orienting behavior in sign-trackers, or on goal-tracking CRs evoked by either a lever-CS or a tone-CS. We conclude that the disruption of reconsolidation by post-retrieval propranolol degrades the emotional/motivational impact of the CS, required for sign-tracking, but leaves the CS–US association intact. The possibility that post-retrieval interventions can reduce the emotional/motivational aspects of memories, without actually erasing them, has important implications for treating maladaptive memories that contribute to some psychiatric disorders.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Nader K, Schafe GE, Le Doux JE. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature. 2000;406:722.
Tronson NC, Wiseman SL, Olausson P, Taylor JR. Bidirectional behavioral plasticity of memory reconsolidation depends on amygdalar protein kinase A. Nat Neurosci. 2006;9:167.
Alberini CM, LeDoux JE. Memory reconsolidation. Curr Biol. 2013;23:R746–50.
Milton AL, Everitt BJ. The psychological and neurochemical mechanisms of drug memory reconsolidation: implications for the treatment of addiction. Eur J Neurosci. 2010;31:2308–19.
Tronson NC, Taylor JR. Addiction: a drug-induced disorder of memory reconsolidation. Curr Opin Neurobiol. 2013;23:573–80.
Lee JL. Reconsolidation: maintaining memory relevance. Trends Neurosci. 2009;32:413–20.
Tronson NC, Taylor JR. Molecular mechanisms of memory reconsolidation. Nat Rev Neurosci. 2007;8:262–75.
Misanin JR, Miller RR, Lewis DJ. Retrograde amnesia produced by electroconvulsive shock after reactivation of a consolidated memory trace. Science. 1968;160:554–5.
Przybyslawski J, Roullet P, Sara SJ. Attenuation of emotional and nonemotional memories after their reactivation: Role of β adrenergic receptors. J Neurosci. 1999;19:6623–8.
Kindt M, Soeter M, Vervliet B. Beyond extinction: erasing human fear responses and preventing the return of fear. Nat Neurosci. 2009;12:256.
Soeter M, Kindt M. Dissociating response systems: erasing fear from memory. Neurobiol Learn Mem. 2010;94:30–41.
Bindra D. A unified interpretation of emotion and motivation. Ann N Y Acad Sci. 1969;159:1071–83.
Flagel SB, Robinson TE. Neurobiological basis of individual variation in stimulus-reward learning. Curr Opin Behav Sci. 2017;13:178–85.
Robinson TE, Yager LM, Cogan ES, Saunders BT. On the motivational properties of reward cues: individual differences. Neuropharmacology. 2014;76:450–9.
Hearst E, Jenkins HM. Sign-tracking: the stimulus-reinforcer relation and directed action. Austin: Monograph of the Psychonomic Society; 1974.
Boakes RA. Performance on learning to associate a stimulus with positive reinforcement. In: Davis H, Hurwitz, H, editors. Operant-Pavlovian interactions. John Wiley & Sons Inc.; Hillsdale, N.J. 1977. p. 67–97.
Robinson TE, Flagel SB. Dissociating the predictive and incentive motivational properties of reward-related cues through the study of individual differences. Biol Psychiatry. 2009;65:869–73.
Saunders BT, Robinson TE. A cocaine cue acts as an incentive stimulus in some but not others: implications for addiction. Biol Psychiatry. 2010;67:730–6.
Yager LM, Robinson TE. Cue-induced reinstatement of food seeking in rats that differ in their propensity to attribute incentive salience to food cues. Behav Brain Res. 2010;214:30–4.
Ahrens AM, Meyer PJ, Ferguson LM, Robinson TE, Aldridge JW. Neural activity in the ventral pallidum encodes variation in the incentive value of a reward cue. J Neurosci. 2016;36:7957–70.
Flagel SB, Cameron CM, Pickup KN, Watson SJ, Akil H, Robinson TE. A food predictive cue must be attributed with incentive salience for it to induce c-fos mRNA expression in cortico-striatal-thalamic brain regions. Neuroscience. 2011;196:80–96.
Yager LM, Pitchers KK, Flagel SB, Robinson TE. Individual variation in the motivational and neurobiological effects of an opioid cue.Neuropsychopharmacology. 2015;40:1269–77.
Olshavsky ME, Song B, Powell DJ, Jones CE, Monfils MH, Lee HJ. Updating appetitive memory during reconsolidation window: critical role of cue-directed behavior and amygdala central nucleus. Front Behav Neurosci. 2013;7:186.
Shumake J, Furgeson-Moreira S, Monfils MH. Predictability and heritability of individual differences in fear learning. Anim Cogn. 2014;17:1207–21.
Meyer PJ, Lovic V, Saunders BT, Yager LM, Flagel SB, Morrow JD, Robinson TE. Quantifying individual variation in the propensity to attribute incentive salience to reward cues. PLoS ONE. 2012;7:e38987.
Saunders BT, Robinson TE. The role of dopamine in the accumbens core in the expression of Pavlovian conditioned responses. Eur J Neurosci. 2012;36:2521–32.
Cleland GG, Davey GC. Autoshaping in the rat: the effects of localizable visual and auditory signals for food. J Exp Anal Behav. 1983;40:47–56.
Meyer PJ, Cogan ES, Robinson TE. The form of a conditioned stimulus can influence the degree to which it acquires incentive motivational properties. PLoS ONE. 2014;9:e98163.
Beckmann JS, Chow JJ. Isolating the incentive salience of reward-associated stimuli: value, choice, and persistence. Learn Mem. 2015;22:116–27.
Singer BF, Bryan MA, Popov P, Scarff R, Carter C, Wright E, Aragona BJ, Robinson TE. The sensory features of a food cue influence its ability to act as an incentive stimulus and evoke dopamine release in the nucleus accumbens core. Learn Mem. 2016;23:595–606.
Milton AL, Lee JL, Everitt BJ. Reconsolidation of appetitive memories for both natural and drug reinforcement is dependent on β-adrenergic receptors. Learn Mem. 2008;15:88–92.
Robinson M, Franklin K. Central but not peripheral beta-adrenergic antagonism blocks reconsolidation for a morphine place preference. Behav Brain Res. 2007;182:129–34.
Vetere G, Piserchia V, Borreca A, Novembre G, Aceti M, Ammassari-Teule M. Reactivating fear memory under propranolol resets pre-trauma levels of dendritic spines in basolateral amygdala but not dorsal hippocampus neurons. Front Behav Neurosci. 2013;7:211
Sandkühler J, Lee J. How to erase memory traces of pain and fear. Trends Neurosci. 2013;36:343–52.
Bouton ME, Moody EW. Memory processes in classical conditioning. Neurosci Biobehav Rev. 2004;28:663–74.
Villain H, Benkahoul A, Drougard A, Lafragette M, Muzotte E, Pech S, Bui E, Brunet A, Birmes P, Roullet P. Effects of propranolol, a β-noradrenergic antagonist, on memory consolidation and reconsolidation in mice. Front Behav Neurosci. 2016;10:49
Cardinal RN, Parkinson JA, Hall J, Everitt BJ. Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci Biobehav Rev. 2002;26:321–52.
Flagel SB, Clark JJ, Robinson TE, Mayo L, Czuj A, Willuhn I, Akers CA, Clinton SM, Phillips PE, Akil H. A selective role for dopamine in stimulus-reward learning. Nature. 2011;469:53–7.
Blaiss CA, Janak PH. Post-training, but not post-reactivation, administration of amphetamine and anisomycin modulates Pavlovian conditioned approach. Neurobiol Learn Mem. 2007;87:644–58.
Reichelt AC, Lee JL. Memory reconsolidation in aversive and appetitive settings. Front Behav Neurosci. 2013;7:118
Pasquariello KZ, Han M, Unal C, Meyer PJ. Adrenergic manipulation inhibits Pavlovian conditioned approach behaviors. Behav Brain Res. 2018;339:278–85.
Fernández RS, Boccia MM, Pedreira ME. The fate of memory: reconsolidation and the case of prediction error. Neurosci Biobehav Rev. 2016;68:423–41.
Duvarci S, Nader K. Characterization of fear memory reconsolidation. J Neurosci. 2004;24:9269–75.
Dunbar AB, Taylor JR. Garcinol blocks the reconsolidation of multiple cocaine-paired cues after a single cocaine-reactivation session. Neuropsychopharmacology. 2017;42:1884.
Finnie PS, Nader K. The role of metaplasticity mechanisms in regulating memory destabilization and reconsolidation. Neurosci Biobehav Rev. 2012;36:1667–707.
Elsey JW, Kindt M. Tackling maladaptive memories through reconsolidation: from neural to clinical science. Neurobiol Learn Mem. 2017;142:108–17.
Delamater AR, Oakeshott S. Learning about multiple attributes of reward in Pavlovian conditioning. Ann N Y Acad Sci. 2007;1104:1–20.
Dayan P, Berridge KC. Model-based and model-free Pavlovian reward learning: revaluation, revision, and revelation. Cogn Affect Behav Neurosci. 2014;14:473–92.
Lesaint F, Sigaud O, Flagel SB, Robinson TE, Khamassi M. Modelling individual differences in the form of pavlovian conditioned approach responses: a dual learning systems approach with factored representations. PLoS Comput Biol. 2014;10:e1003466.
Robinson MJF, Berridge KC. Instant transformation of learned repulsion into motivational “wanting”. Curr Biol. 2013;23:282–9.
Zhang J, Berridge KC, Tindell AJ, Smith KS, Aldridge JW. A neural computational model of incentive salience. PLoS Comput Biol. 2009;5:e1000437.
Mahabir M, Tucholka A, Shin LM, Etienne P, Brunet A. Emotional face processing in post-traumatic stress disorder after reconsolidation impairment using propranolol: a pilot fMRI study. J Anxiety Disord. 2015;36:127–33.
Brunet A, Poundja J, Tremblay J, Bui É, Thomas É, Orr SP, Azzoug A, Birmes P, Pitman RK. Trauma reactivation under the influence of propranolol decreases posttraumatic stress symptoms and disorder: 3 open-label trials. J Clin Psychopharmacol. 2011;31:547–50.
Lonergan MH, Saumier D, Tremblay J, Kieffer B, Brown TG, Brunet A. Reactivating addiction-related memories under propranolol to reduce craving: a pilot randomized controlled trial. J Behav Ther Exp Psychiatry. 2016;50:245–9.
Saladin ME, Gray K, Abbott T, LaRowe S, McRae-Clark A, DeSantis S, Baker N, Back S, Hartwell K, Brady KT. Post-retrieval propranolol may alter reconsolidation of trauma memory in individuals with PTSD and comorbid alcohol dependence. Drug Alcohol Depend. 2014;140:e193.
Kolber AJ. Therapeutic forgetting: the legal and ethical implications of memory dampening. Vand L Rev. 2006;59:1559.
Kolber A. Neuroethics: give memory-altering drugs a chance. Nature. 2011;476:275–6.
Acknowledgements
We thank Alex Kawa, Elizabeth O’Donnell, and Meghan Zechmeister for their assistance with behavioral testing, and Kyle Pitchers and Joshua Haight for assistance with the immunohistochemistry. Research supported by grant P01 DA031656 to TER. Authors report no financial interests or potential conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interest
This work was supported by the National Institutes of Health Grant P01 DA031656 to TER. All authors declare no financial and non-financial competing interests. The authors declare no conflict of interest.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cogan, E.S., Shapses, M.A., Robinson, T.E. et al. Disrupting reconsolidation: memory erasure or blunting of emotional/motivational value?. Neuropsychopharmacol 44, 399–407 (2019). https://doi.org/10.1038/s41386-018-0082-0
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41386-018-0082-0
This article is cited by
-
Negative allosteric modulation of mGlu7 disrupts fear memory reconsolidation and glutamatergic signaling in rat and human brain tissue
Molecular Psychiatry (2026)
-
Drug memory reconsolidation: from molecular mechanisms to the clinical context
Translational Psychiatry (2023)
-
Aging mice show impaired memory updating in the novel OUL updating paradigm
Neuropsychopharmacology (2020)
-
Interfering with emotional processing resources upon associative threat memory reactivation does not affect memory retention
Scientific Reports (2019)