Abstract
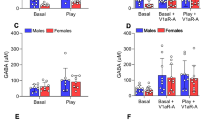
Social play is a highly rewarding behavior displayed mostly during the juvenile period. We recently showed that vasopressin V1a receptor (V1aR) blockade in the lateral septum (LS) enhances social play in male juvenile rats, but reduces it in females. Here, we determined whether the LS-AVP system modulates dopamine (DA) and/or norepinephrine (NE) neurotransmission in the LS to regulate social play behavior in sex-specific ways. Using microdialysis combined with retrodialysis, we demonstrated that both LS-AVP administration and social play exposure increased extracellular LS-DA release in females, but not in males. Pharmacological blockade of LS-DA receptors reduced social play in both sexes, but required a higher dose in females. This suggests that baseline LS-DA release is sufficient for social play in males, while increased LS-DA release is necessary for social play in females. Administration of a V1aR antagonist into the LS inhibited the social play-induced increase in extracellular LS-DA release in females. Furthermore, co-administration of the DA agonist apomorphine prevented the LS-V1aR blockade-induced decrease in social play in females. This suggests that LS-V1aR blockade reduces social play in females by dampening the rise in LS-DA release. Extracellular LS-NE release was enhanced in response to pharmacological manipulations of the LS-AVP system and to social play in males and/or females, but pharmacological blockade or stimulation of LS-NE receptors did not alter social play in either sex. Overall, we define a mechanism by which the LS-AVP system alters LS-DA neurotransmission differently in males than females resulting in the sex-specific regulation of juvenile social play behavior.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Trezza V, Baarendse PJ, Vanderschuren LJ. The pleasures of play: pharmacological insights into social reward mechanisms. Trends Pharmacol Sci. 2010;31:463–9.
Vanderschuren LJ, Achterberg EJ, Trezza V. The neurobiology of social play and its rewarding value in rats. Neurosci Biobehav Rev. 2016;70:86–105.
Panksepp J. The ontogeny of play in rats. Dev Psychobiol. 1981;14:327–32.
Pellis SM, Pellis VC. Book The PlayfulBrain: Venturing to the Limits of Neuroscience. Oxford: Oneworld Publications; 2009.
Jordan R. Social play and autistic spectrum disorders: a perspective on theory, implications and educational approaches. Autism. 2003;7:347–60.
Kohls G, Chevallier C, Troiani V, Schultz RT. Social ‘wanting’ dysfunction in autism: neurobiological underpinnings and treatment implications. J Neurodev Disord. 2012;4:10.
Chevallier C, Kohls G, Troiani V, Brodkin ES, Schultz RT. The social motivation theory of autism. Trends Cogn Sci. 2012;16:231–9.
Rutter M, Caspi A, Moffitt TE. Using sex differences in psychopathology to study causal mechanisms: unifying issues and research strategies. J Child Psychol Psychiatry. 2003;44:1092–115.
Achterberg EJM, van Kerkhof LWM, Servadio M, van Swieten MM, Houwing DJ, Aalderink M, Driel NV, Trezza V, Vanderschuren LJMJ. Contrasting roles of dopamine and noradrenaline in the motivational properties of social play behavior in rats. Neuropsychopharmacology. 2016;41:858–68.
Achterberg EJM, Van Kerkhof LWM, Damsteegt R, Trezza V, Vanderschuren LJMJ. Methylphenidate and atomoxetine inhibit social play behavior through prefrontal and subcortical limbic mechanisms in rats. J Neurosci. 2015;35:161–169.
Manduca A, Servadio M, Damsteegt R, Campolongo P, Vanderschuren LJ, Trezza V. Dopaminergic neurotransmission in the nucleus accumbens modulates social play behavior in rats. Neuropsychopharmacology. 2016;41:2215–23.
Veenema AH, Bredewold R, De Vries GJ. Sex-specific modulation of juvenile social play by vasopressin. Psychoneuroendocrinology. 2013;38:2554–61.
Bredewold R, Smith CJ, Dumais KM, Veenema AH. Sex-specific modulation of juvenile social play behavior by vasopressin and oxytocin depends on social context. Front Behav Neurosci. 2014;16.8:216.
Bredewold R, Schiavo JK, van der Hart M, Verreij M, Veenema AH. Dynamic changes in extracellular release of GABA and glutamate in the lateral septum during social play behavior in juvenile rats: implications for sex-specific regulation of social play behavior. Neuroscience. 2015;307:117–27.
Lindvall O. Mesencephalic dopaminergic afferents to the lateral septal nucleus of the rat. Brain Res. 1975;87:89–95.
Lindvall O, Stenevi U. Dopamine and noradrenaline neurons projecting to the septal area in the rat. Cell Tiss Res. 1978;190:383–407.
Assaf SY, Miller JJ. Excitatory action of the mesolimbic dopamine system on septal neurones. Brain Res. 1977;129:353–60.
Joëls M, Urban IJ. Monoamine-induced responses in lateral septal neurons: influence of iontophoretically applied vasopressin. Brain Res. 1985;344:120–6.
Sheehan TP, Chambers RA, Russell DS. Regulation of affect by the lateral septum: implications for neuropsychiatry. Brain Res Brain Res Rev. 2004;46:71–117.
De Vries GJ, Buijs RM. The origin of the vasopressinergic and oxytocinergic innervation of the rat brain with special reference to the lateral septum. Brain Res. 1983;273:307–17.
De Vries GJ, Best W, Sluiter AA. The influence of androgens on the development of a sex difference in the vasopressinergic innervation of the rat lateral septum. Brain Res. 1983;284:377–80.
Caffé AR, van Leeuwen FW, Luiten PG. Vasopressin cells in the medial amygdala of the rat project to the lateral septum and ventral hippocampus. J Comp Neurol. 1987;261:237–52.
Lukas M, Bredewold R, Landgraf R, Neumann ID, Veenema AH. Early life stress impairs social recognition due to a blunted response of vasopressin release within the septum of adult male rats. Psychoneuroendocrinology. 2011;36:843–53.
Manning M, Stoev S, Chini B, Durroux T, Mouillac B, Guillon G. Peptide and non-peptide agonists and antagonists for the vasopressin and oxytocin V1a, V1b, V2 and OT receptors: research tools and potential therapeutic agents. Prog Brain Res. 2008;170:473–512.
Dwarkasing JT, Witkamp RF, Boekschoten MV, Ter Laak MC, Heins MS, van Norren K. Increased hypothalamic serotonin turnover in inflammation-induced anorexia. BMC Neurosci. 2016;17:26.
Gulia KK, Kumar VM, Mallick HN. Role of the lateral septal noradrenergic system in the elaboration of male sexual behavior in rats. Pharmacol Biochem Behav. 2002;72:817–23.
Okada S, Yamaguchi N. Possible role of adrenoceptors in the hypothalamic paraventricular nucleus in corticotropin-releasing factor-induced sympatho-adrenomedullary outflow in rats. Auton Neurosci. 2017;203:74–80.
Pal GK, Pal P, Raj SS, Mohan M. Modulation of feeding and drinking behaviour by catecholamines injected into nucleus caudatus in rats. Indian J Physiol Pharmacol. 2001;45:172–80.
Scotti MA, Lee G, Gammie SC. Maternal defense is modulated by beta adrenergic receptors in lateral septum in mice. Behav Neurosci. 2011;125:434–45.
Yilmaz MS, Myer BS, Feleder C, Millington WR. Blockade of {alpha}-adrenergic receptors in the preoptic area/anterior hypothalamus prevents lipopolysaccharide evoked hypotension. FASEB J. 2008;22:1227.8.
Veenema AH, Neumann ID. Maternal separation enhances offensive play-fighting, basal corticosterone and hypothalamic vasopressin mRNA expression in juvenile male rats. Psychoneuroendocrinology. 2009;34:463–7.
Reddy IA, Pino JA, Weikop P, Osses N, Sørensen G, Bering T, Valle C, Bluett RJ, Erreger K, Wortwein G, Reyes JG, Graham D, Stanwood GD, Hackett TA, Patel S, Fink-Jensen A, Torres GE, Galli A. Glucagon-like peptide 1 receptor activation regulates cocaine actions and dopamine homeostasis in the lateral septum by decreasing arachidonic acid levels. Transl Psychiatry. 2016;6:e809.
Sotomayor R, Forray MI, Gysling K. Acute morphine administration increases extracellular DA levels in the rat lateral septum by decreasing the GABAergic inhibitory tone in the ventral tegmental area. J Neurosci Res. 2005;81:132–9.
Olds J, Milner P. Positive reinforcement produced by electrical stimulation of septal area and other regions of rat brain. J Comp Physiol Psychol. 1954;47:419–27.
Bishop MP, Elder ST, Heath RG. Intracranial self-stimulation in man. Science. 1963;140:394–6.
Luo AH, Tahsili-Fahadan P, Wise RA, Lupica CR, Aston-Jones G. Linking context with reward: a functional circuit from hippocampal CA3 to ventral tegmental area. Science. 2011;333:353–7.
Sartor GC, Aston-Jones GS. A septal-hypothalamic pathway drives orexin neurons, which is necessary for conditioned cocaine preference. J Neurosci. 2012;32:4623–31.
Northcutt KV, Nguyen JM. Female juvenile play elicits Fos expression in dopaminergic neurons of the VTA. Behav Neurosci. 2014;128:178–86.
Smith CJ, Poehlmann ML, Li S, Ratnaseelan AM, Bredewold R, Veenema AH. Age and sex differences in oxytocin and vasopressin V1a receptor binding densities in the rat brain: focus on the social decision-making network. Brain Struct Funct. 2017;222:981–1006.
Veenema AH, Bredewold R, De Vries GJ. Vasopressin regulates social recognition in juvenile and adult rats of both sexes, but in sex- and age-specific ways. Horm Behav. 2012;61:50–56.
Ishizawa H, Tabakoff B, Mefford IN, Hoffman PL. Reduction of arginine vasopressin binding sites in mouse lateral septum by treatment with 6-hydroxydopamine. Brain Res. 1990;507:189–94.
DiBenedictis BT, Nussbaum ER, Cheung HK, Veenema AH. Quantitative mapping reveals age and sex differences in vasopressin, but not oxytocin, immunoreactivity in the rat social behavior neural network. J Comp Neurol. 2017;525:2549–70.
De Vries GJ, Panzica GC. Sexual differentiation of central vasopressin and vasotocin systems in vertebrates: different mechanisms, similar endpoints. Neuroscience. 2006;138:947–55.
Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press, San Diego 2007
Engelmann M, Ludwig M, Landgraf R. Microdialysis administration of vasopressin and vasopressin antagonists into the septum during pole-jumping behavior in rats. Behav Neural Biol. 1992;58:51–7.
Acknowledgements
We thank Jennifer Schiavo and Michelle Verreij for technical assistance, Dr. Maurice Manning for providing the V1aR antagonist, and members of the Veenema lab for input in previous versions of the manuscript. This research was supported by NSF IOS1253386 and NIH R01MH102456 to AHV.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Bredewold, R., Nascimento, N.F., Ro, G.S. et al. Involvement of dopamine, but not norepinephrine, in the sex-specific regulation of juvenile socially rewarding behavior by vasopressin. Neuropsychopharmacol 43, 2109–2117 (2018). https://doi.org/10.1038/s41386-018-0100-2
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41386-018-0100-2
This article is cited by
-
Vasopressin regulates social play behavior in sex-specific ways through glutamate modulation in the lateral septum
Neuropsychopharmacology (2025)
-
The positive reinforcing effects of cocaine and opposite-sex social contact: roles of biological sex and estrus
Psychopharmacology (2025)