Abstract
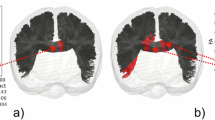
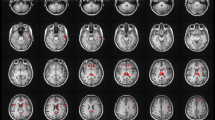
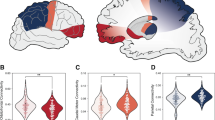
Catatonia is characterized by motor, affective and behavioral abnormalities. To date, the specific role of white matter (WM) abnormalities in schizophrenia spectrum disorders (SSD) patients with catatonia is largely unknown. In this study, diffusion magnetic resonance imaging (dMRI) data were collected from 111 right-handed SSD patients and 28 healthy controls. Catatonic symptoms were examined on the Northoff Catatonia Rating Scale (NCRS). We used whole-brain tract-based spatial statistics (TBSS), tractometry (along tract statistics using TractSeg) and graph analytics (clustering coefficient—CCO, local betweenness centrality—BC) to provide a framework of specific WM microstructural abnormalities underlying catatonia in SSD. Following a categorical approach, post hoc analyses showed differences in fractional anisotrophy (FA) measured via tractometry in the corpus callosum, corticospinal tract and thalamo-premotor tract as well as increased CCO as derived by graph analytics of the right superior parietal cortex (SPC) and left caudate nucleus in catatonic patients (NCRS total score ≥ 3; n = 30) when compared to non-catatonic patients (NCRS total score = 0; n = 29). In catatonic patients according to DSM-IV-TR (n = 43), catatonic symptoms were associated with FA variations (tractometry) of the left corticospinal tract and CCO of the left orbitofrontal cortex, primary motor cortex, supplementary motor area and putamen. This study supports the notion that structural reorganization of WM bundles connecting orbitofrontal/parietal, thalamic and striatal regions contribute to catatonia in SSD patients.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Northoff G, Koch A, Wenke J, Eckert J, Boker H, Pflug B, et al. Catatonia as a psychomotor syndrome: a rating scale and extrapyramidal motor symptoms. Mov Disord: Off J Mov Disord Soc. 1999;14:404–16.
Fink M. Rediscovering catatonia: the biography of a treatable syndrome. Acta Psychiatr Scand Suppl. 2013;441:1–47.
Fink M, Shorter E, Taylor MA. Catatonia is not schizophrenia: Kraepelin’s error and the need to recognize catatonia as an independent syndrome in medical nomenclature. Schizophr Bull. 2010;36:314–20.
Francis A, Fink M, Appiani F, Bertelsen A, Bolwig TG, Braunig P, et al. Catatonia in diagnostic and statistical manual of mental disorders, fifth edition. J Ect. 2010;26:246–7.
Wilson JE, Niu K, Nicolson SE, Levine SZ, Heckers S. The diagnostic criteria and structure of catatonia. Schizophrenia Res. 2015;164:256–62.
Kirby GH. The catatonic syndrome and its relation to manic-depressive insanity. J Nerv Ment Dis. 1913;40:694–704.
Lange J. Katatonische Erscheinungen im Rahmen manisch-depressiver Erkrankungen. Berlin: Springer; 1922.
Hirjak D, Wolf RC, Northoff G GABA and negative affect-catatonia as model of RDoC-based investigation in psychiatry. Schizophr Bull. 2019;45:1168–69.
Hirjak D, Kubera KM, Wolf RC, Northoff G. Going back to Kahlbaum’s psychomotor (and GABAergic) origins: is catatonia more than just a motor and dopaminergic syndrome? Schizophr Bull. 2020;46:272–85.
Hirjak D, Rashidi M, Kubera KM, Northoff G, Fritze S, Schmitgen MM, et al. Multimodal magnetic resonance imaging data fusion reveals distinct patterns of abnormal brain structure and function in catatonia. Schizophr Bull. 2020;46:202–10.
Hirjak D, Kubera KM, Northoff G, Fritze S, Bertolino AL, Topor CE, et al. Cortical contributions to distinct symptom dimensions of catatonia. Schizophr Bull. 2019;45:1184–94.
Walther S, Schappi L, Federspiel A, Bohlhalter S, Wiest R, Strik W, et al. Resting-state hyperperfusion of the supplementary motor area in Catatonia. Schizophr Bull. 2017;43:972–81.
Walther S, Stegmayer K, Federspiel A, Bohlhalter S, Wiest R, Viher PV. Aberrant hyperconnectivity in the motor system at rest is linked to motor abnormalities in schizophrenia spectrum disorders. Schizophr Bull. 2017;43:982–92.
Walther S, Stegmayer K, Wilson JE, Heckers S. Structure and neural mechanisms of catatonia. Lancet Psychiatry. 2019;6:610–19.
Ellul P, Choucha W. Neurobiological approach of catatonia and treatment perspectives. Front psychiatry. 2015;6:182.
Rogers JP, Pollak TA, Blackman G, David AS. Catatonia and the immune system: a review. Lancet Psychiatry. 2019;6:620–30.
Janova H, Arinrad S, Balmuth E, Mitjans M, Hertel J, Habes M, et al. Microglia ablation alleviates myelin-associated catatonic signs in mice. J Clin Invest. 2018;128:734–45.
Hagemeyer N, Goebbels S, Papiol S, Kastner A, Hofer S, Begemann M, et al. A myelin gene causative of a catatonia-depression syndrome upon aging. EMBO Mol Med. 2012;4:528–39.
Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. NeuroImage. 2006;31:1487–505.
Wassermann D, Rathi Y, Bouix S, Kubicki M, Kikinis R, Shenton M, et al. White matter bundle registration and population analysis based on Gaussian processes. Inf Process Med Imaging. 2011;22:320–32.
Yeatman JD, Dougherty RF, Rykhlevskaia E, Sherbondy AJ, Deutsch GK, Wandell BA, et al. Anatomical properties of the arcuate fasciculus predict phonological and reading skills in children. J Cogn Neurosci. 2011;23:3304–17.
Wasserthal J, Neher PF, Hirjak D, Maier-Hein KH. Combined tract segmentation and orientation mapping for bundle-specific tractography. Med Image Anal. 2019;58:101559.
Tsujino N, Nemoto T, Yamaguchi T, Katagiri N, Tohgi N, Ikeda R, et al. Cerebral blood flow changes in very-late-onset schizophrenia-like psychosis with catatonia before and after successful treatment. Psychiatry Clin Neurosci. 2011;65:600–3.
Lauer M, Schirrmeister H, Gerhard A, Ellitok E, Beckmann H, Reske SN, et al. Disturbed neural circuits in a subtype of chronic catatonic schizophrenia demonstrated by F-18-FDG-PET and F-18-DOPA-PET. J Neural Transm (Vienna). 2001;108:661–70.
Goch CJ, Stieltjes B, Henze R, Hering J, Poustka L, Meinzer HP, et al. Quantification of changes in language-related brain areas in autism spectrum disorders using large-scale network analysis. Int J Comp Assisted Radiol Surg. 2014;9:357–65.
Freeman LC. A set of measures of centrality based on betweenness. Sociometry. 1977;40:31–40.
Hirjak D, Thomann PA, Northoff G, Kubera KM, Wolf RC NCR-Skala - Deutsche Version der Northoff Catatonia Rating Scale (NCRS-dv) - Ein validiertes Messinstrument zur Erfassung katatoner Symptome. Der Nervenarzt. 2016:(im Druck).
Ungvari GS, Caroff SN, Gerevich J. The catatonia conundrum: evidence of psychomotor phenomena as a symptom dimension in psychotic disorders. Schizophrenia Bull. 2010;36:231–8.
Heckers S, Tandon R, Bustillo J. Catatonia in the DSM-shall we move or not? Schizophr Bull. 2010;36:205–7.
Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113.
Sass H, Wittchen HU, Zaudig MIH. Diagnostisches und Statistisches Manual Psychischer Störungen DSM-IV-TR: Textrevision. Hogrefe Verlag; Auflage: 1 (1. Januar 2003); 2003.
Leucht S, Samara M, Heres S, Patel MX, Furukawa T, Cipriani A, et al. Dose equivalents for second-generation antipsychotic drugs: the classical mean dose method. Schizophr Bull. 2015;41:1397–402.
Dazzan P, Morgan KD, Orr KG, Hutchinson G, Chitnis X, Suckling J, et al. The structural brain correlates of neurological soft signs in AESOP first-episode psychoses study. Brain. 2004;127:143–53.
Gay O, Plaze M, Oppenheim C, Mouchet-Mages S, Gaillard R, Olie JP, et al. Cortex morphology in first-episode psychosis patients with neurological soft signs. Schizophr Bull. 2013;39:820–9.
Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. Fsl. Neuroimage. 2012;62:782–90.
Woolrich MW, Jbabdi S, Patenaude B, Chappell M, Makni S, Behrens T, et al. Bayesian analysis of neuroimaging data in FSL. Neuroimage. 2009;45:S173–86.
Veraart J, Novikov DS, Christiaens D, Ades-Aron B, Sijbers J, Fieremans E. Denoising of diffusion MRI using random matrix theory. Neuroimage. 2016;142:394–406.
Kellner E, Dhital B, Kiselev VG, Reisert M. Gibbs-ringing artifact removal based on local subvoxel-shifts. Magn Reson Med: Off J Soc Magn Reson Med / Soc Magn Reson Med. 2016;76:1574–81.
Andersson JLR, Sotiropoulos SN. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. Neuroimage. 2016;125:1063–78.
Tustison NJ, Avants BB, Cook PA, Zheng Y, Egan A, Yushkevich PA, et al. N4ITK: improved N3 bias correction. IEEE Trans Med Imaging. 2010;29:1310–20.
Smith SM. Fast robust automated brain extraction. Hum brain Mapp. 2002;17:143–55.
Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5:143–56.
Bach M, Laun FB, Leemans A, Tax CMW, Biessels GJ, Stieltjes B, et al. Methodological considerations on tract-based spatial statistics (TBSS). NeuroImage. 2014;100:358–69.
Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. NeuroImage. 2009;44:83–98.
Kelly S, Jahanshad N, Zalesky A, Kochunov P, Agartz I, Alloza C, et al. Widespread white matter microstructural differences in schizophrenia across 4322 individuals: results from the ENIGMA Schizophrenia DTI Working Group. Mol Psychiatry. 2018;23:1261–69.
Wasserthal J, Neher P, Maier-Hein KH. TractSeg - Fast and accurate white matter tract segmentation. Neuroimage. 2018;183:239–53.
Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum brain Mapp. 2002;15:1–25.
Yeatman JD, Dougherty RF, Myall NJ, Wandell BA, Feldman HM. Tract profiles of white matter properties: automating fiber-tract quantification. PLoS One. 2012;7:e49790.
Neher PF, Descoteaux M, Houde JC, Stieltjes B, Maier-Hein KH. Strengths and weaknesses of state of the art fiber tractography pipelines-A comprehensive in-vivo and phantom evaluation study using Tractometer. Med Image Anal. 2015;26:287–305.
Rubinov M, Bullmore E. Fledgling pathoconnectomics of psychiatric disorders. Trends Cogn Sci. 2013;17:641–7.
Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci USA. 2000;97:11050–5.
Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: inflation, flattening, and a surface-based coordinate system. NeuroImage. 1999;9:195–207.
Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. NeuroImage. 1999;9:179–94.
Fischl B, van der Kouwe A, Destrieux C, Halgren E, Segonne F, Salat DH, et al. Automatically parcellating the human cerebral cortex. Cereb Cortex. 2004;14:11–22.
Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage. 2006;31:968–80.
Segonne F, Dale AM, Busa E, Glessner M, Salat D, Hahn HK, et al. A hybrid approach to the skull stripping problem in MRI. NeuroImage. 2004;22:1060–75.
Smith RE, Tournier JD, Calamante F, Connelly A. The effects of SIFT on the reproducibility and biological accuracy of the structural connectome. Neuroimage. 2015;104:253–65.
Barnes J, Ridgway GR, Bartlett J, Henley SM, Lehmann M, Hobbs N, et al. Head size, age and gender adjustment in MRI studies: a necessary nuisance? Neuroimage. 2010;53:1244–55.
Hyatt CS, Owens MM, Crowe ML, Carter NT, Lynam DR, Miller JD. The quandary of covarying: a brief review and empirical examination of covariate use in structural neuroimaging studies on psychological variables. Neuroimage. 2020;205:116225.
Payoux P, Boulanouar K, Sarramon C, Fabre N, Descombes S, Galitsky M, et al. Cortical motor activation in akinetic schizophrenic patients: a pilot functional MRI study. Mov Disord: Off J Mov Disord Soc. 2004;19:83–90.
Mamah D, Ji A, Rutlin J, Shimony JS. White matter integrity in schizophrenia and bipolar disorder: Tract- and voxel-based analyses of diffusion data from the Connectom scanner. Neuroimage Clin. 2019;21:101649.
Huttlova J, Kikinis Z, Kerkovsky M, Bouix S, Vu MA, Makris N, et al. Abnormalities in myelination of the superior cerebellar peduncle in patients with schizophrenia and deficits in movement sequencing. Cerebellum. 2014;13:415–24.
Gulani V, Webb AG, Duncan ID, Lauterbur PC. Apparent diffusion tensor measurements in myelin-deficient rat spinal cords. Magn Reson Med. 2001;45:191–5.
Kubicki M, McCarley RW, Shenton ME. Evidence for white matter abnormalities in schizophrenia. Curr Opin psychiatry. 2005;18:121–34.
Kubicki M, Westin CF, McCarley RW, Shenton ME. The application of DTI to investigate white matter abnormalities in schizophrenia. Ann N. Y Acad Sci. 2005;1064:134–48.
Kubicki M, Park H, Westin CF, Nestor PG, Mulkern RV, Maier SE, et al. DTI and MTR abnormalities in schizophrenia: analysis of white matter integrity. Neuroimage. 2005;26:1109–18.
D’Agati E, Casarelli L, Pitzianti MB, Pasini A. Overflow movements and white matter abnormalities in ADHD. Prog Neuro-Psychopharmacol Biol psychiatry. 2010;34:441–5.
Poggi G, Boretius S, Mobius W, Moschny N, Baudewig J, Ruhwedel T, et al. Cortical network dysfunction caused by a subtle defect of myelination. Glia. 2016;64:2025–40.
Muetzel RL, Collins PF, Mueller BA, Lim AMS, Luciana KO. M. The development of corpus callosum microstructure and associations with bimanual task performance in healthy adolescents. NeuroImage. 2008;39:1918–25.
Porter R. Lemon R. Corticospinal Function and Voluntary Movement. Oxford, NY: Clarendon Press, Oxford University Press; 1993.
Witham CL, Fisher KM, Edgley SA, Baker SN. Corticospinal Inputs to Primate Motoneurons Innervating the Forelimb from Two Divisions of Primary Motor Cortex and Area 3a. J Neurosci: Off J Soc Neurosci. 2016;36:2605–16.
Bopp MHA, Zollner R, Jansen A, Dietsche B, Krug A, Kircher TTJ. White matter integrity and symptom dimensions of schizophrenia: a diffusion tensor imaging study. Schizophr Res. 2017;184:59–68.
Wu L, Calhoun VD, Jung RE, Caprihan A. Connectivity-based whole brain dual parcellation by group ICA reveals tract structures and decreased connectivity in schizophrenia. Hum brain Mapp. 2015;36:4681–701.
Forssberg H, Eliasson AC, Kinoshita H, Johansson RS, Westling G. Development of human precision grip. I: Basic coordination of force. Exp brain Res. 1991;85:451–7.
Nashef A, Cohen O, Israel Z, Harel R, Prut Y. Cerebellar shaping of motor cortical firing is correlated with timing of motor actions. Cell Rep. 2018;23:1275–85.
Nashef A, Rapp H, Nawrot MP, Prut Y. Area-specific processing of cerebellar-thalamo-cortical information in primates. Biol Cybern 2018;112:141–52.
Viher PV, Stegmayer K, Federspiel A, Bohlhalter S, Wiest R, Walther S. Altered diffusion in motor white matter tracts in psychosis patients with catatonia. Schizophr Res. 2020;12:S0920-9964:30120–1. https://doi.org/10.1016/j.schres.2020.03.017. epub ahead of print.
Northoff G. What catatonia can tell us about “top-down modulation”: a neuropsychiatric hypothesis. Behav Brain Sci. 2002;25:555–77.
Lara AH, Cunningham JP, Churchland MM. Different population dynamics in the supplementary motor area and motor cortex during reaching. Nat Commun. 2018;9:2754.
Scheuerecker J, Ufer S, Kapernick M, Wiesmann M, Bruckmann H, Kraft E, et al. Cerebral network deficits in post-acute catatonic schizophrenic patients measured by fMRI. J Psychiatr Res. 2009;43:607–14.
Rasmussen SA, Mazurek MF, Rosebush PI. Catatonia: Our current understanding of its diagnosis, treatment and pathophysiology. World J Psychiatry. 2016;6:391–98.
Acknowledgements
We are grateful to all the participants and their families for their time and interest in this study.
Author information
Authors and Affiliations
Contributions
DH, RCW and KMK: Design of the study. JW, KHM-H, PFN: Data analysis. DH, SF, AH, LSG and HT: Data collection. DH, RCW, JW, GN, KMK: Interpretation of the results, writing and manuscript revision.
Corresponding author
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Wasserthal, J., Maier-Hein, K.H., Neher, P.F. et al. Multiparametric mapping of white matter microstructure in catatonia. Neuropsychopharmacol. 45, 1750–1757 (2020). https://doi.org/10.1038/s41386-020-0691-2
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41386-020-0691-2
This article is cited by
-
Common and unique white matter fractional anisotropy patterns in patients with schizophrenia with medication-resistant auditory verbal hallucinations: a retrospective tract-based spatial statistics study
Schizophrenia (2025)
-
Sensori- and psychomotor abnormalities, psychopathological symptoms and functionality in schizophrenia-spectrum disorders: a network analytic approach
Schizophrenia (2025)
-
White matter microstructural and macrostructural profiles during midlife reveal sex differences between men and women at different menopausal stages
Scientific Reports (2025)
-
Deciphering white matter microstructural alterations in catatonia according to ICD-11: replication and machine learning analysis
Molecular Psychiatry (2025)
-
Associations among bipolar II depression white matter subgroups, inflammation, symptoms and childhood maltreatment
Nature Mental Health (2025)