Abstract
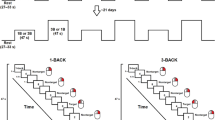
Ordering of information is a critical component that underlies several cognitive functions. Prefrontal theta–gamma coupling (TGC) is a neurophysiologic measure associated with ordering of information during the performance of a working memory task (N-back). Little is known about the relationship between TGC and ordering during other cognitive tasks or whether the relationship between TGC and ordering of information is independent of clinical condition. This study aimed to determine whether the relationship between TGC and ordering of information exists independent of a task and its timing, and whether this relationship is the same in different clinical conditions. A total of 311 participants were assessed using a neuropsychological battery that included the N-back during which TGC was measured; two other tasks that also require ordering; and three tests that do not require ordering. All non-N-back tasks were completed several days separate from the N-back by a mean interval (SD) of 5.14 (8.03). Our three hypotheses were that TGC during the N-back task would be associated with performance on N-Back and other cognitive tasks that also require ordering, but not with performance on cognitive tasks that do not require ordering; and that these relationships will be independent of clinical diagnosis. Multivariate linear regression results show that TGC was associated with performance on the ordering tasks but not the non-ordering tasks. In addition, there was no interaction between TGC and diagnosis. Our study is the first to demonstrate that TGC is a neurophysiologic measure of ordering information across several cognitive tasks that require ordering, and this TGC-ordering relationship is stable over time even when several days separate the measurement of TGC and the performance of the ordering tasks. Our results also show that this relationship is independent of clinical diagnosis, supporting the brain–behavior nature of this relationship. These results highlight the importance of TGC in ordering-based cognition, and suggest that TGC could be a valid target for interventions that aim to enhance this function across cognitive tasks and clinical conditions.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Ward G, Tan L, Grenfell-Essam R. Examining the relationship between free recall and immediate serial recall: the effects of list length and output order. J Exp Psychol Learn Mem Cogn. 2010;36:1207.
Grenfell-Essam R, Ward G. Examining the relationship between free recall and immediate serial recall: the role of list length, strategy use, and test expectancy. J Mem Lang. 2012;67:106–48.
Eichenbaum H. A cortical–hippocampal system for declarative memory. Nat Rev Neurosci. 2000;1:41.
Bott J-B, Muller M-A, Jackson J, Aubert J, Cassel J-C, Mathis C, et al. Spatial reference memory is associated with modulation of theta–gamma coupling in the dentate gyrus. Cereb Cortex. 2016;26:3744–53.
Hannesson D, Vacca G, Howland J, Phillips A. Medial prefrontal cortex is involved in spatial temporal order memory but not spatial recognition memory in tests relying on spontaneous exploration in rats. Behav Brain Res. 2004;153:273–85.
Lashley KS. The problem of serial order in behavior. Oxford, UK: Bobbs-Merrill; 1951.
Hurlstone MJ, Hitch GJ, Baddeley AD. Memory for serial order across domains: an overview of the literature and directions for future research. Psychol Bull. 2014;140:339.
Tort AB, Komorowski RW, Manns JR, Kopell NJ, Eichenbaum H. Theta–gamma coupling increases during the learning of item–context associations. Proc Natl Acad Sci. 2009;106:20942–47.
Rajji TK, Zomorrodi R, Barr MS, Blumberger DM, Mulsant BH, Daskalakis ZJ. Ordering information in working memory and modulation of gamma by theta oscillation in humans. Cereb Cortex. 2017;27:1482–90.
Canolty RT, Knight RT. The functional role of cross-frequency coupling. Trends Cogn Sci. 2010;14:506–15.
Lisman JE, Jensen O. The theta-gamma neural code. Neuron. 2013;77:1002–16.
Shirvalkar PR, Rapp PR, Shapiro ML. Bidirectional changes to hippocampal theta–gamma comodulation predict memory for recent spatial episodes. Proc Natl Acad Sci. 2010;107:7054–59.
Jensen O, Tesche CD. Frontal theta activity in humans increases with memory load in a working memory task. Eur J Neurosci. 2002;15:1395–99.
Goodman MS, Kumar S, Zomorrodi R, Ghazala Z, Cheam ASM, Barr MS, et al. Theta–gamma coupling and working memory in Alzheimer’s dementia and mild cognitive impairment. Front Aging Neurosci. 2018;10:101.
Rajji TK, Bowie CR, Herrmann N, Pollock BG, Bikson M, Blumberger DM, et al. Design and Rationale of the PACt-MD randomized clinical trial: prevention of Alzheimer’s dementia with cognitive remediation plus transcranial direct current stimulation in mild cognitive impairment and depression. J Alzheimer’s Dis. 2020:1–29. In press.
Association AP. Diagnostic and statistical manual of mental disorders (DSM-5®). American Psychiatric Association Publishing; 2013.
Montgomery SA, Åsberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–89.
First M, Williams J, Karg R, Spitzer R. Structured clinical interview for DSM-5—research version (SCID-5 for DSM-5, research version; SCID-5-RV). Arlington, VA: American Psychiatric Association; 2015.
Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98.
Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatrics Soc. 2005;53:695–99.
Gronwall D. Paced auditory serial-addition task: a measure of recovery from concussion. Percept Mot Skills. 1977;44:367–73.
Strauss E, Sherman EM, Spreen O. A compendium of neuropsychological tests: administration, norms, and commentary. American Chemical Society; Journal of clinical and experimental neuropsychology; 2006.
Arbuthnott K, Frank J. Trail making test, part B as a measure of executive control: validation using a set-switching paradigm. J Clin Exp Neuropsychol. 2000;22:518–28.
Kaplan E, Goodglass H, Weintraub S. Boston naming test. Pro-Ed Inc; 2001.
Wechsler D. Wechsler Adult Intelligence Scale–Fourth Edition (WAIS–IV). San Antonio, TX: The Psychological Corporation; 2008.
Benton AL, Hamsher K, Varney NR, Spreen O. Judgment of line orientation. New York: Oxford University Press; 1983.
Fisher NJ, Tierney MC, Snow GW, Szalai JP. Odd/even short forms of the Boston naming test: preliminary geriatric norms. Clin Neuropsychol. 1999;13:359–64.
Barr MS, Farzan F, Rajji TK, Voineskos AN, Blumberger DM, Arenovich T, et al. Can repetitive magnetic stimulation improve cognition in schizophrenia? Pilot data from a randomized controlled trial. Biol Psychiatry. 2013;73:510–17.
Axmacher N, Henseler MM, Jensen O, Weinreich I, Elger CE, Fell J. Cross-frequency coupling supports multi-item working memory in the human hippocampus. Proc Natl Acad Sci. 2010;107:3228–33.
Rajji TK, Zomorrodi R, Barr MS, Blumberger DM, Mulsant BH, Daskalakis ZJ. Ordering information in working memory and modulation of gamma by theta oscillations in humans. Cereb Cortex. 2017;27:1482–90.
Cardinal KS, Wilson SM, Giesser BS, Drain AE, Sicotte NL. A longitudinal fMRI study of the paced auditory serial addition task. Mult Scler. 2008;14:465–71.
Audoin B, Ibarrola D, Duong MVA, Pelletier J, Confort-Gouny S, Malikova I, et al. Functional MRI study of PASAT in normal subjects. Magn Reson Mater Phys Biol Med. 2005;18:96–102.
Moll J, de Oliveira-Souza R, Moll FT, Bramati IE, Andreiuolo PA. The cerebral correlates of set-shifting—an fMRI study of the trail making test. Arquivos De Neuro-Psiquiatria. 2002;60:900–5.
Zakzanis KK, Mraz Z, Graham S. An fMRI study of the Trail Making Test. Neuropsychologia. 2005;43:1878–86.
Rajji TK, Sun Y, Zomorrodi-Moghaddam R, Farzan F, Blumberger DM, Mulsant BH, et al. PAS-induced potentiation of cortical evoked activity in the dorsolateral prefrontal cortex. Neuropsychopharmacology. 2013;38:2545–52.
Noda Y, Zomorrodi R, Saeki T, Rajji TK, Blumberger DM, Daskalakis ZJ, et al. Resting-state EEG gamma power and theta–gamma coupling enhancement following high-frequency left dorsolateral prefrontal rTMS in patients with depression. Clin Neurophysiol. 2017;128:424–32.
Reinhart RM, Nguyen JA. Working memory revived in older adults by synchronizing rhythmic brain circuits. Nat Neurosci. 2019;1:820–7.
Acknowledgements
This work has been made possible by Brain Canada through the Canada Brain Research Fund, with the financial support of Health Canada and the Chagnon Family; Canada Foundation for Innovation (#25861, PI: TKR); Canadian Institutes of Health Research (#244041, PI: TKR); the Ontario Ministry of Research and Innovation (# ER14-10-004, PI: TKR); and the Joan and Clifford Hatch Foundation. The authors would like to thank the entire PACt-MD Study Group: Benoit H. Mulsant, Tarek K. Rajji, Nathan Herrmann, Bruce G. Pollock, Lillian Lourenco, Daniel M. Blumberger, Christopher R. Bowie, Meryl Butters, Corinne Fischer, Alastair Flint, Damian Gallagher, Angela Golas, Ariel Graff, James L. Kennedy, Sanjeev Kumar, Linda Mah, Shima Ovaysikia, Mark. Rapoport, Kevin Thorpe, Nicolaas P.L.G. Verhoeff, Aristotle N. Voineskos.
Author information
Authors and Affiliations
Consortia
Contributions
HB analyzed the data and wrote the paper. HB and TKR reviewed the first draft. MSG, RZ, SK, and TKR developed the analysis methods. BHM, TKR, DMB, and CRB developed the design of the parent study. BHM, TKR, DMB, CEF, AF, LM, NH, SK, and CRB contributed to the recruitment and assessment of participants. ZJD, AV, MAB, BGP, and all other co-authors aided in interpretation of the results and editing of the paper. All authors reviewed and approved the final paper.
Corresponding author
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Brooks, H., Goodman, M.S., Bowie, C.R. et al. Theta–gamma coupling and ordering information: a stable brain–behavior relationship across cognitive tasks and clinical conditions. Neuropsychopharmacol. 45, 2038–2047 (2020). https://doi.org/10.1038/s41386-020-0759-z
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41386-020-0759-z
This article is cited by
-
Modulation of brain oscillations by continuous theta burst stimulation in patients with insomnia
Translational Psychiatry (2025)
-
Cognitive function based on theta-gamma coupling vs. clinical diagnosis in older adults with mild cognitive impairment with or without major depressive disorder
Translational Psychiatry (2024)
-
Neurophysiological and other features of working memory in older adults at risk for dementia
Cognitive Neurodynamics (2024)
-
The olfactory bulb coordinates the ventral hippocampus–medial prefrontal cortex circuit during spatial working memory performance
The Journal of Physiological Sciences (2022)
-
Cognitive control, interference inhibition, and ordering of information during working memory in younger and older healthy adults
GeroScience (2022)