Abstract
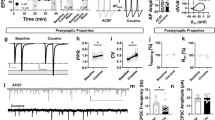
Cocaine use and abstinence induce long-term synaptic alterations in the excitatory input to nucleus accumbens (NAc) medium spiny neurons (MSNs). The NAc regulates reward-related behaviors through two parallel projections to the ventral pallidum (VP)—originating in D1 or D2-expressing MSNs (D1-MSNs→VP; D2-MSNs→VP). The activity of these projections depends on their excitatory synaptic inputs, but it is not known whether and how abstinence from cocaine affects the excitatory transmission to D1-MSNs→VP and D2-MSNs→VP. Here we examined different forms of cocaine-induced synaptic plasticity in the inputs from the basolateral amygdala (BLA) and medial prefrontal cortex (mPFC) to NAc D1-MSNs→VP and putative D2-MSNs→VP (pD2-MSNs→VP) in the core and shell subcompartments of the NAc. We used the whole-cell patch-clamp technique to record excitatory postsynaptic currents from D1-tdTomato mice injected with ChR2 in either the BLA or the mPFC and retrograde tracer (RetroBeads) in the VP. We found that cocaine conditioned place preference (CPP) followed by abstinence potentiated the excitatory input from the BLA and mPFC to both D1-MSNs→VP and pD2-MSNs→VP. Interestingly, while the strengthening of the inputs to D1-MSNs→VP was of postsynaptic origin and manifested as increased AMPA to NMDA ratio, in pD2-MSNs→VP plasticity was predominantly presynaptic and was detected as changes in the paired-pulse ratio and coefficient of variation. Lastly, some of the changes were sex-specific. Overall our data show that abstinence from cocaine changes the excitatory inputs to both D1-MSNs→VP and pD2-MSNs→VP but with different mechanisms. This may help understand how circuits converging into the VP change after cocaine exposure.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Kalivas PW, O’Brien C. Drug addiction as a pathology of staged neuroplasticity. Neuropsychopharmacology. 2008;33:166–80. https://doi.org/10.1038/sj.npp.1301564
Chiamulera C, Piva A, Abraham WC. Glutamate receptors and metaplasticity in addiction. Curr Opin Pharmacol. 2021;56:39–45. https://doi.org/10.1016/j.coph.2020.09.005
Kauer JA, Malenka RC. Synaptic plasticity and addiction. Nat Rev Neurosci. 2007;8:844–58. https://doi.org/10.1038/nrn2234
Pickens CL, Airavaara M, Theberge F, Fanous S, Hope BT, Shaham Y. Neurobiology of the incubation of drug craving. Trends Neurosci. 2011;34:411–20. https://doi.org/10.1016/j.tins.2011.06.001
Volkow ND, Morales M. The brain on drugs: from reward to addiction. Cell. 2015;162:712–25. https://doi.org/10.1016/j.cell.2015.07.046
Kourrich S, Rothwell PE, Klug JR, Thomas MJ. Cocaine experience controls bidirectional synaptic plasticity in the nucleus accumbens. J Neurosci. 2007;27:7921–8. https://doi.org/10.1523/JNEUROSCI.1859-07.2007
Gipson CD, Kupchik YM, Shen H, Reissner KJ, Thomas CA, Kalivas PW. Relapse induced by cues predicting cocaine depends on rapid, transient synaptic potentiation. Neuron. 2013;77:867–72. https://doi.org/10.1016/j.neuron.2013.01.005
Zinsmaier AK, Dong Y, Huang YH. Cocaine-induced projection-specific and cell type-specific adaptations in the nucleus accumbens. Mol Psychiatry. 2021. https://doi.org/10.1038/s41380-021-01112-2
Pascoli V, Terrier J, Espallergues J, Valjent E, O’Connor EC, Lüscher C. Contrasting forms of cocaine-evoked plasticity control components of relapse. Nature. 2014;509:459–64. https://doi.org/10.1038/nature13257
Ortinski PI, Vassoler FM, Carlson GC, Pierce RC. Temporally dependent changes in cocaine-induced synaptic plasticity in the nucleus accumbens shell are reversed by D1-like dopamine receptor stimulation. Neuropsychopharmacology. 2012;37:1671–82. https://doi.org/10.1038/npp.2012.12
Dobi A, Seabold GK, Christensen CH, Bock R, Alvarez VA. Cocaine-induced plasticity in the nucleus accumbens is cell specific and develops without prolonged withdrawal. J Neurosci. 2011;31:1895–904. https://doi.org/10.1523/JNEUROSCI.5375-10.2011
Bock R, Shin JH, Kaplan AR, Dobi A, Markey E, Kramer PF, et al. Strengthening the accumbal indirect pathway promotes resilience to compulsive cocaine use. Nat Neurosci. 2013;16:632–8. https://doi.org/10.1038/nn.3369
Thomas MJ, Beurrier C, Bonci A, Malenka RC. Long-term depression in the nucleus accumbens: a neural correlate of behavioral sensitization to cocaine. Nat Neurosci. 2001;4:1217–23. https://doi.org/10.1038/nn757
Gerfen CR, Surmeier DJ. Modulation of striatal projection systems by dopamine. Annu Rev Neurosci. 2011;34:441–66. https://doi.org/10.1146/annurev-neuro-061010-113641
Kupchik YM, Brown RM, Heinsbroek JA, Lobo MK, Schwartz DJ, Kalivas PW. Coding the direct/indirect pathways by D1 and D2 receptors is not valid for accumbens projections. Nat Neurosci. 2015;18:1230–2. https://doi.org/10.1038/nn.4068
MacAskill AF, Cassel JM, Carter AG. Cocaine exposure reorganizes cell type- and input-specific connectivity in the nucleus accumbens. Nat Neurosci. 2014;17:1198–207. https://doi.org/10.1038/nn.3783
Lobo MK, Covington HE, Chaudhury D, Friedman AK, Sun H, Damez-Werno D, et al. Cell type-specific loss of BDNF signaling mimics optogenetic control of cocaine reward. Science. 2010;330:385–90. https://doi.org/10.1126/science.1188472
Heimer L, Zahm DS, Churchill L, Kalivas PW, Wohltmann C. Specificity in the projection patterns of accumbal core and shell in the rat. Neuroscience. 1991;41:89–125. https://doi.org/10.1016/0306-4522(91)90202-y
Zahm DS, Heimer L. Two transpallidal pathways originating in the rat nucleus accumbens. J Comp Neurol. 1990;302:437–46. https://doi.org/10.1002/cne.903020302
Smith KS, Tindell AJ, Aldridge JW, Berridge KC. Ventral pallidum roles in reward and motivation. Behav Brain Res. 2009;196:155–67. https://doi.org/10.1016/j.bbr.2008.09.038
Root DH, Melendez RI, Zaborszky L, Napier TC. The ventral pallidum: subregion-specific functional anatomy and roles in motivated behaviors. Prog Neurobiol. 2015;130:29–70. https://doi.org/10.1016/j.pneurobio.2015.03.005
Creed M, Ntamati NR, Chandra R, Lobo MK, Lüscher C. Convergence of reinforcing and anhedonic cocaine effects in the ventral pallidum. Neuron. 2016;92:214–26. https://doi.org/10.1016/j.neuron.2016.09.001
Inbar K, Levi LA, Bernat N, Odesser T, Inbar D, Kupchik YM. Cocaine dysregulates dynorphin modulation of inhibitory neurotransmission in the ventral pallidum in a cell-type-specific manner. J Neurosci. 2020;40:1321–31. https://doi.org/10.1523/JNEUROSCI.1262-19.2019
Baimel C, McGarry LM, Carter AG. The projection targets of medium spiny neurons govern cocaine-evoked synaptic plasticity in the nucleus accumbens. Cell Rep. 2019;28:2256–63. https://doi.org/10.1016/j.celrep.2019.07.074. e3
Heinsbroek JA, Bobadilla A-C, Dereschewitz E, Assali A, Chalhoub RM, Cowan CW, et al. Opposing regulation of cocaine seeking by glutamate and GABA neurons in the ventral pallidum. Cell Rep. 2020;30:2018–27. https://doi.org/10.1016/j.celrep.2020.01.023. e3
Heinsbroek JA, Neuhofer DN, Griffin WC, Siegel GS, Bobadilla A-C, Kupchik YM, et al. Loss of plasticity in the D2-accumbens pallidal pathway promotes cocaine seeking. J Neurosci. 2017;37:757–67. https://doi.org/10.1523/JNEUROSCI.2659-16.2016
Pardo-Garcia TR, Garcia-Keller C, Penaloza T, Richie CT, Pickel J, Hope BT, et al. Ventral pallidum is the primary target for accumbens D1 projections driving cocaine seeking. J Neurosci. 2019;39:2041–51. https://doi.org/10.1523/JNEUROSCI.2822-18.2018
Ben-Shaul Y. OptiMouse: a comprehensive open source program for reliable detection and analysis of mouse body and nose positions. BMC Biol. 2017;15:41 https://doi.org/10.1186/s12915-017-0377-3
Paxinos G, Franklin KBJ. The mouse brain in stereotaxic coordinates. 2nd ed. San Diego, CA, USA: Academic Press; 2001.
Britt JP, Benaliouad F, McDevitt RA, Stuber GD, Wise RA, Bonci A. Synaptic and behavioral profile of multiple glutamatergic inputs to the nucleus accumbens. Neuron. 2012;76:790–803. https://doi.org/10.1016/j.neuron.2012.09.040
Phillipson OT, Griffiths AC. The topographic order of inputs to nucleus accumbens in the rat. Neuroscience. 1985;16:275–96. https://doi.org/10.1016/0306-4522(85)90002-8
Scudder SL, Baimel C, Macdonald EE, Carter AG. Hippocampal-evoked feedforward inhibition in the nucleus accumbens. J Neurosci. 2018;38:9091–104. https://doi.org/10.1523/JNEUROSCI.1971-18.2018
O’Donnell P, Grace AA. Physiological and morphological properties of accumbens core and shell neurons recorded in vitro. Synapse. 1993;13:135–60. https://doi.org/10.1002/syn.890130206
Ade KK, Wan Y, Chen M, Gloss B, Calakos N. An improved BAC transgenic fluorescent reporter line for sensitive and specific identification of striatonigral medium spiny neurons. Front Syst Neurosci. 2011;5:32 https://doi.org/10.3389/fnsys.2011.00032
Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng L-J, Shaham Y, et al. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature. 2008;454:118–21. https://doi.org/10.1038/nature06995
Lee BR, Ma Y-Y, Huang YH, Wang X, Otaka M, Ishikawa M, et al. Maturation of silent synapses in amygdala-accumbens projection contributes to incubation of cocaine craving. Nat Neurosci. 2013;16:1644–51. https://doi.org/10.1038/nn.3533
Terrier J, Lüscher C, Pascoli V. Cell-type specific insertion of GluA2-lacking AMPARs with cocaine exposure leading to sensitization, cue-induced seeking, and incubation of craving. Neuropsychopharmacology. 2016;41:1779–89. https://doi.org/10.1038/npp.2015.345
Shukla A, Beroun A, Panopoulou M, Neumann PA, Grant SG, Olive MF, et al. Calcium-permeable AMPA receptors and silent synapses in cocaine-conditioned place preference. EMBO J. 2017;36:458–74. https://doi.org/10.15252/embj.201695465
Mameli M, Halbout B, Creton C, Engblom D, Parkitna JR, Spanagel R, et al. Cocaine-evoked synaptic plasticity: persistence in the VTA triggers adaptations in the NAc. Nat Neurosci. 2009;12:1036–41. https://doi.org/10.1038/nn.2367
McCutcheon JE, Wang X, Tseng KY, Wolf ME, Marinelli M. Calcium-permeable AMPA receptors are present in nucleus accumbens synapses after prolonged withdrawal from cocaine self-administration but not experimenter-administered cocaine. J Neurosci. 2011;31:5737–43. https://doi.org/10.1523/JNEUROSCI.0350-11.2011
Hollmann M, Hartley M, Heinemann S. Ca2+ permeability of KA-AMPA-gated glutamate receptor channels depends on subunit composition. Science. 1991;252:851–3. https://doi.org/10.1126/science.1709304
Bochet P, Audinat E, Lambolez B, Crépel F, Rossier J, Iino M, et al. Subunit composition at the single-cell level explains functional properties of a glutamate-gated channel. Neuron. 1994;12:383–8. https://doi.org/10.1016/0896-6273(94)90279-8
Liu SQ, Cull-Candy SG. Synaptic activity at calcium-permeable AMPA receptors induces a switch in receptor subtype. Nature. 2000;405:454–8. https://doi.org/10.1038/35013064
Liu SJ, Cull-Candy SG. Activity-dependent change in AMPA receptor properties in cerebellar stellate cells. J Neurosci. 2002;22:3881–9. 20026392
Parnas H, Segel LA. Ways to discern the presynaptic effect of drugs on neurotransmitter release. J Theor Biol. 1982;94:923–41. https://doi.org/10.1016/0022-5193(82)90087-x
Faber DS, Korn H. Applicability of the coefficient of variation method for analyzing synaptic plasticity. Biophys J 1991;60:1288–94. https://doi.org/10.1016/S0006-3495(91)82162-2
Brock JA, Thomazeau A, Watanabe A, Li SSY, Sjöström PJ. A practical guide to using CV analysis for determining the locus of synaptic plasticity. Front Synaptic Neurosci. 2020;12:11 https://doi.org/10.3389/fnsyn.2020.00011
Alonso-Caraballo Y, Fetterly TL, Jorgensen ET, Nieto AM, Brown TE, Ferrario CR. Sex specific effects of “junk-food” diet on calcium permeable AMPA receptors and silent synapses in the nucleus accumbens core. Neuropsychopharmacology. 2021;46:569–78. https://doi.org/10.1038/s41386-020-0781-1
Alonso-Caraballo Y, Jorgensen ET, Brown T, Ferrario CR. Functional and structural plasticity contributing to obesity: roles for sex, diet, and individual susceptibility. Curr Opin Behav Sci. 2018;23:160–70. https://doi.org/10.1016/j.cobeha.2018.06.014
Wickens MM, Kirkland JM, Knouse MC, McGrath AG, Briand LA. Sex-specific role for prefrontal cortical protein interacting with C kinase 1 in cue-induced cocaine seeking. Addict Biol. 2021;26:e13051 https://doi.org/10.1111/adb.13051
LaRese TP, Rheaume BA, Abraham R, Eipper BA, Mains RE. Sex-specific gene expression in the mouse nucleus accumbens before and after cocaine exposure. J Endocr Soc. 2019;3:468–87. https://doi.org/10.1210/js.2018-00313
Engeln M, Mitra S, Chandra R, Gyawali U, Fox ME, Dietz DM, et al. Sex-specific role for Egr3 in nucleus accumbens D2-medium spiny neurons following long-term abstinence from cocaine self-administration. Biol Psychiatry. 2020;87:992–1000. https://doi.org/10.1016/j.biopsych.2019.10.019
Zachry JE, Nolan SO, Brady LJ, Kelly SJ, Siciliano CA, Calipari ES. Sex differences in dopamine release regulation in the striatum. Neuropsychopharmacology. 2021;46:491–9. https://doi.org/10.1038/s41386-020-00915-1
Ritchie JL, Walters JL, Galliou JMC, Christian RJ, Qi S, Savenkova MI, et al. Basolateral amygdala corticotropin-releasing factor receptor type 1 regulates context-cocaine memory strength during reconsolidation in a sex-dependent manner. Neuropharmacology. 2021;200:108819 https://doi.org/10.1016/j.neuropharm.2021.108819
Kupchik YM, Scofield MD, Rice KC, Cheng K, Roques BP, Kalivas PW. Cocaine dysregulates opioid gating of GABA neurotransmission in the ventral pallidum. J Neurosci. 2014;34:1057–66. https://doi.org/10.1523/JNEUROSCI.4336-13.2014
Curran EJ, Watson SJ. Dopamine receptor mRNA expression patterns by opioid peptide cells in the nucleus accumbens of the rat: a double in situ hybridization study. J Comp Neurol. 1995;361:57–76. https://doi.org/10.1002/cne.903610106
Le Moine C, Bloch B. D1 and D2 dopamine receptor gene expression in the rat striatum: sensitive cRNA probes demonstrate prominent segregation of D1 and D2 mRNAs in distinct neuronal populations of the dorsal and ventral striatum. J Comp Neurol. 1995;355:418–26. https://doi.org/10.1002/cne.903550308
Lu XY, Ghasemzadeh MB, Kalivas PW. Expression of D1 receptor, D2 receptor, substance P and enkephalin messenger RNAs in the neurons projecting from the nucleus accumbens. Neuroscience. 1998;82:767–80. https://doi.org/10.1016/s0306-4522(97)00327-8
Iino Y, Sawada T, Yamaguchi K, Tajiri M, Ishii S, Kasai H, et al. Dopamine D2 receptors in discrimination learning and spine enlargement. Nature. 2020;579:555–60. https://doi.org/10.1038/s41586-020-2115-1
Soares-Cunha C, Coimbra B, David-Pereira A, Borges S, Pinto L, Costa P, et al. Activation of D2 dopamine receptor-expressing neurons in the nucleus accumbens increases motivation. Nat Commun. 2016;7:11829 https://doi.org/10.1038/ncomms11829
Soares-Cunha C, de Vasconcelos NAP, Coimbra B, Domingues AV, Silva JM, Loureiro-Campos E, et al. Nucleus accumbens medium spiny neurons subtypes signal both reward and aversion. Mol Psychiatry. 2020;25:3241–55. https://doi.org/10.1038/s41380-019-0484-3
Scofield MD, Heinsbroek JA, Gipson CD, Kupchik YM, Spencer S, Smith ACW, et al. The nucleus accumbens: mechanisms of addiction across drug classes reflect the importance of glutamate homeostasis. Pharmacol Rev. 2016;68:816–71. https://doi.org/10.1124/pr.116.012484
Gibson GD, Millan EZ, McNally GP. The nucleus accumbens shell in reinstatement and extinction of drug seeking. Eur J Neurosci. 2019;50:2014–22. https://doi.org/10.1111/ejn.14084
Hearing MC, Jedynak J, Ebner SR, Ingebretson A, Asp AJ, Fischer RA, et al. Reversal of morphine-induced cell-type-specific synaptic plasticity in the nucleus accumbens shell blocks reinstatement. Proc Natl Acad Sci USA. 2016;113:757–62. https://doi.org/10.1073/pnas.1519248113
Stuber GD, Sparta DR, Stamatakis AM, van Leeuwen WA, Hardjoprajitno JE, Cho S, et al. Excitatory transmission from the amygdala to nucleus accumbens facilitates reward seeking. Nature. 2011;475:377–80. https://doi.org/10.1038/nature10194
Stefanik MT, Kupchik YM, Kalivas PW. Optogenetic inhibition of cortical afferents in the nucleus accumbens simultaneously prevents cue-induced transient synaptic potentiation and cocaine-seeking behavior. Brain Struct Funct. 2016;221:1681–9. https://doi.org/10.1007/s00429-015-0997-8
Stefanik MT, Kalivas PW. Optogenetic dissection of basolateral amygdala projections during cue-induced reinstatement of cocaine seeking. Front Behav Neurosci. 2013;7:213 https://doi.org/10.3389/fnbeh.2013.00213
Ma Y-Y, Lee BR, Wang X, Guo C, Liu L, Cui R, et al. Bidirectional modulation of incubation of cocaine craving by silent synapse-based remodeling of prefrontal cortex to accumbens projections. Neuron. 2014;83:1453–67. https://doi.org/10.1016/j.neuron.2014.08.023
Bamford NS, Wang W. Corticostriatal plasticity in the nucleus accumbens core. J Neurosci Res. 2019;97:1559–78. https://doi.org/10.1002/jnr.24494
Domingo-Rodriguez L, Ruiz de Azua I, Dominguez E, Senabre E, Serra I, Kummer S, et al. A specific prelimbic-nucleus accumbens pathway controls resilience versus vulnerability to food addiction. Nat Commun. 2020;11:782 https://doi.org/10.1038/s41467-020-14458-y
Blakemore S-J, Robbins TW. Decision-making in the adolescent brain. Nat Neurosci. 2012;15:1184–91. https://doi.org/10.1038/nn.3177
Hearing M. Prefrontal-accumbens opioid plasticity: implications for relapse and dependence. Pharmacol Res. 2019;139:158–65. https://doi.org/10.1016/j.phrs.2018.11.012
Ambroggi F, Ishikawa A, Fields HL, Nicola SM. Basolateral amygdala neurons facilitate reward-seeking behavior by exciting nucleus accumbens neurons. Neuron. 2008;59:648–61. https://doi.org/10.1016/j.neuron.2008.07.004
Marchant NJ, Kaganovsky K, Shaham Y, Bossert JM. Role of corticostriatal circuits in context-induced reinstatement of drug seeking. Brain Res. 2015;1628:219–32. https://doi.org/10.1016/j.brainres.2014.09.004
Levi LA, Inbar K, Nachshon N, Bernat N, Gatterer A, Inbar D, et al. Projection-specific potentiation of ventral pallidal glutamatergic outputs after abstinence from cocaine. J Neurosci. 2020;40:1276–85. https://doi.org/10.1523/JNEUROSCI.0929-19.2019
Gong W, Neill D, Justice JB. 6-Hydroxydopamine lesion of ventral pallidum blocks acquisition of place preference conditioning to cocaine. Brain Res. 1997;754:103–12. https://doi.org/10.1016/s0006-8993(97)00059-0
Pribiag H, Shin S, Wang EH-J, Sun F, Datta P, Okamoto A, et al. Ventral pallidum DRD3 potentiates a pallido-habenular circuit driving accumbal dopamine release and cocaine seeking. Neuron. 2021;109:2165–82. https://doi.org/10.1016/j.neuron.2021.05.002. e10
Skoubis PD, Maidment NT. Blockade of ventral pallidal opioid receptors induces a conditioned place aversion and attenuates acquisition of cocaine place preference in the rat. Neuroscience. 2003;119:241–9. https://doi.org/10.1016/s0306-4522(03)00121-0
Kupchik YM, Prasad AA. Ventral pallidum cellular and pathway specificity in drug seeking. Neurosci Biobehav Rev. 2021;131:373–86. https://doi.org/10.1016/j.neubiorev.2021.09.007
Suska A, Lee BR, Huang YH, Dong Y, Schlüter OM. Selective presynaptic enhancement of the prefrontal cortex to nucleus accumbens pathway by cocaine. Proc Natl Acad Sci USA 2013;110:713–8. https://doi.org/10.1073/pnas.1206287110
Ferrario CR, Loweth JA, Milovanovic M, Ford KA, Galiñanes GL, Heng L-J, et al. Alterations in AMPA receptor subunits and TARPs in the rat nucleus accumbens related to the formation of Ca2+-permeable AMPA receptors during the incubation of cocaine craving. Neuropharmacology. 2011;61:1141–51. https://doi.org/10.1016/j.neuropharm.2011.01.021
Kamboj SK, Swanson GT, Cull-Candy SG. Intracellular spermine confers rectification on rat calcium-permeable AMPA and kainate receptors. J Physiol. 1995;486:297–303. https://doi.org/10.1113/jphysiol.1995.sp020812. Pt 2
O’Donnell P, Grace AA. Synaptic interactions among excitatory afferents to nucleus accumbens neurons: hippocampal gating of prefrontal cortical input. J Neurosci. 1995;15:3622–39.
Zucker RS. Short-term synaptic plasticity. Annu Rev Neurosci. 1989;12:13–31. https://doi.org/10.1146/annurev.ne.12.030189.000305
Katz B, Miledi R. The role of calcium in neuromuscular facilitation. J Physiol. 1968;195:481–92. https://doi.org/10.1113/jphysiol.1968.sp008469
Hearing M, Graziane N, Dong Y, Thomas MJ. Opioid and psychostimulant plasticity: targeting overlap in nucleus accumbens glutamate signaling. Trends Pharmacol Sci. 2018;39:276–94. https://doi.org/10.1016/j.tips.2017.12.004
Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci. 2009;10:561–72. https://doi.org/10.1038/nrn2515
Kupchik YM, Moussawi K, Tang X-C, Wang X, Kalivas BC, Kolokithas R, et al. The effect of N-acetylcysteine in the nucleus accumbens on neurotransmission and relapse to cocaine. Biol Psychiatry. 2012;71:978–86. https://doi.org/10.1016/j.biopsych.2011.10.024
Shen H, Scofield MD, Boger H, Hensley M, Kalivas PW. Synaptic glutamate spillover due to impaired glutamate uptake mediates heroin relapse. J Neurosci. 2014;34:5649–57. https://doi.org/10.1523/JNEUROSCI.4564-13.2014
Trantham-Davidson H, LaLumiere RT, Reissner KJ, Kalivas PW, Knackstedt LA. Ceftriaxone normalizes nucleus accumbens synaptic transmission, glutamate transport, and export following cocaine self-administration and extinction training. J Neurosci. 2012;32:12406–10. https://doi.org/10.1523/JNEUROSCI.1976-12.2012
Moussawi K, Zhou W, Shen H, Reichel CM, See RE, Carr DB, et al. Reversing cocaine-induced synaptic potentiation provides enduring protection from relapse. Proc Natl Acad Sci USA. 2011;108:385–90. https://doi.org/10.1073/pnas.1011265108
Wu X, Shi M, Wei C, Yang M, Liu Y, Liu Z, et al. Potentiation of synaptic strength and intrinsic excitability in the nucleus accumbens after 10 days of morphine withdrawal. J Neurosci Res. 2012;90:1270–83. https://doi.org/10.1002/jnr.23025
Manzoni O, Michel JM, Bockaert J. Metabotropic glutamate receptors in the rat nucleus accumbens. Eur J Neurosci. 1997;9:1514–23. https://doi.org/10.1111/j.1460-9568.1997.tb01506.x
Robbe D, Alonso G, Chaumont S, Bockaert J, Manzoni OJ. Role of p/q-Ca2+ channels in metabotropic glutamate receptor 2/3-dependent presynaptic long-term depression at nucleus accumbens synapses. J Neurosci. 2002;22:4346–56. 20026420
Johnson LR, Aylward RL, Hussain Z, Totterdell S. Input from the amygdala to the rat nucleus accumbens: its relationship with tyrosine hydroxylase immunoreactivity and identified neurons. Neuroscience. 1994;61:851–65. https://doi.org/10.1016/0306-4522(94)90408-1
Sesack SR, Pickel VM. Prefrontal cortical efferents in the rat synapse on unlabeled neuronal targets of catecholamine terminals in the nucleus accumbens septi and on dopamine neurons in the ventral tegmental area. J Comp Neurol. 1992;320:145–60. https://doi.org/10.1002/cne.903200202
Floresco SB, Blaha CD, Yang CR, Phillips AG. Dopamine D1 and NMDA receptors mediate potentiation of basolateral amygdala-evoked firing of nucleus accumbens neurons. J Neurosci. 2001;21:6370–6.
Wang W, Dever D, Lowe J, Storey GP, Bhansali A, Eck EK, et al. Regulation of prefrontal excitatory neurotransmission by dopamine in the nucleus accumbens core. J Physiol. 2012;590:3743–69. https://doi.org/10.1113/jphysiol.2012.235200
Robbe D, Kopf M, Remaury A, Bockaert J, Manzoni OJ. Endogenous cannabinoids mediate long-term synaptic depression in the nucleus accumbens. Proc Natl Acad Sci USA. 2002;99:8384–8. https://doi.org/10.1073/pnas.122149199
Deroche MA, Lassalle O, Castell L, Valjent E, Manzoni OJ. Cell-type- and endocannabinoid-specific synapse connectivity in the adult nucleus accumbens core. J Neurosci. 2020;40:1028–41. https://doi.org/10.1523/JNEUROSCI.1100-19.2019
Araque A, Castillo PE, Manzoni OJ, Tonini R. Synaptic functions of endocannabinoid signaling in health and disease. Neuropharmacology. 2017;124:13–24. https://doi.org/10.1016/j.neuropharm.2017.06.017
Fourgeaud L, Mato S, Bouchet D, Hémar A, Worley PF, Manzoni OJ. A single in vivo exposure to cocaine abolishes endocannabinoid-mediated long-term depression in the nucleus accumbens. J Neurosci. 2004;24:6939–45. https://doi.org/10.1523/JNEUROSCI.0671-04.2004
McCutcheon JE, Loweth JA, Ford KA, Marinelli M, Wolf ME, Tseng KY. Group I mGluR activation reverses cocaine-induced accumulation of calcium-permeable AMPA receptors in nucleus accumbens synapses via a protein kinase C-dependent mechanism. J Neurosci. 2011;31:14536–41. https://doi.org/10.1523/JNEUROSCI.3625-11.2011
Huang YH, Lin Y, Mu P, Lee BR, Brown TE, Wayman G, et al. In vivo cocaine experience generates silent synapses. Neuron. 2009;63:40–7. https://doi.org/10.1016/j.neuron.2009.06.007
Brown TE, Lee BR, Mu P, Ferguson D, Dietz D, Ohnishi YN, et al. A silent synapse-based mechanism for cocaine-induced locomotor sensitization. J Neurosci. 2011;31:8163–74. https://doi.org/10.1523/JNEUROSCI.0016-11.2011
Grueter BA, Robison AJ, Neve RL, Nestler EJ, Malenka RC. ∆FosB differentially modulates nucleus accumbens direct and indirect pathway function. Proc Natl Acad Sci USA. 2013;110:1923–8. https://doi.org/10.1073/pnas.1221742110
Costa RP, Mizusaki BEP, Sjöström PJ, van Rossum MCW. Functional consequences of pre- and postsynaptic expression of synaptic plasticity. Philos Trans R Soc Lond B, Biol Sci. 2017;372. https://doi.org/10.1098/rstb.2016.0153
Matsui A, Alvarez VA. Cocaine inhibition of synaptic transmission in the ventral pallidum is pathway-specific and mediated by serotonin. Cell Rep. 2018;23:3852–63. https://doi.org/10.1016/j.celrep.2018.05.076
Calipari ES, Juarez B, Morel C, Walker DM, Cahill ME, Ribeiro E, et al. Dopaminergic dynamics underlying sex-specific cocaine reward. Nat Commun. 2017;8:13877 https://doi.org/10.1038/ncomms13877
Author contributions
Conceptualization, KI and YMK.; Methodology, KI and YMK; Formal analysis, KI and YMK; Investigation, KI, LAL and YMK; Writing—original draft, KI; Writing—review & Editing, KI and YMK; Visualization, KI; Supervision, YMK; Project Administration, KI, and YMK; Funding Acquisition, YMK, KI, and LAL.
Funding
This study was supported by the Israeli Science Foundation (grants 1381/15 and 1117/21 to YMK), by the Warshafsky Medical Research Scholarship awarded to KI and LAL, by the U.S.-Israel Binational Science Foundation Prof. Rahamimoff Travel Grant to KI and by the Foulkes Foundation Fellowship to LAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Inbar, K., Levi, L.A. & Kupchik, Y.M. Cocaine induces input and cell-type-specific synaptic plasticity in ventral pallidum-projecting nucleus accumbens medium spiny neurons. Neuropsychopharmacol. 47, 1461–1472 (2022). https://doi.org/10.1038/s41386-022-01285-6
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41386-022-01285-6
This article is cited by
-
Acetazolamide inhibition of carbonic anhydrase 4 reverses opioid-induced synaptic rearrangements in nucleus accumbens and reduces drug-seeking behavior
Neuropsychopharmacology (2026)
-
Medial prefrontal cortex circuit dynamics involved in stage-specific addiction
Brain Structure and Function (2025)
-
NPAS4 supports cocaine-conditioned cues in rodents by controlling the cell type-specific activation balance in the nucleus accumbens
Nature Communications (2024)
-
Transcriptome profiling of the ventral pallidum reveals a role for pallido-thalamic neurons in cocaine reward
Molecular Psychiatry (2022)