Abstract
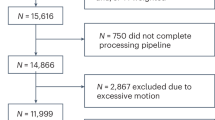
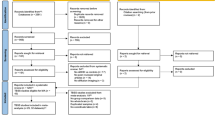
We sought to identify resting-state characteristics related to attention deficit/hyperactivity disorder, both as a categorical diagnosis and as a trait feature, using large-scale samples which were processed according to a standardized pipeline. In categorical analyses, we considered 1301 subjects with diagnosed ADHD, contrasted against 1301 unaffected controls (total N = 2602; 1710 males (65.72%); mean age = 10.86 years, sd = 2.05). Cases and controls were 1:1 nearest neighbor matched on in-scanner motion and key demographic variables and drawn from multiple large cohorts. Associations between ADHD-traits and resting-state connectivity were also assessed in a large multi-cohort sample (N = 10,113). ADHD diagnosis was associated with less anticorrelation between the default mode and salience/ventral attention (B = 0.009, t = 3.45, p-FDR = 0.004, d = 0.14, 95% CI = 0.004, 0.014), somatomotor (B = 0.008, t = 3.49, p-FDR = 0.004, d = 0.14, 95% CI = 0.004, 0.013), and dorsal attention networks (B = 0.01, t = 4.28, p-FDR < 0.001, d = 0.17, 95% CI = 0.006, 0.015). These results were robust to sensitivity analyses considering comorbid internalizing problems, externalizing problems and psychostimulant medication. Similar findings were observed when examining ADHD traits, with the largest effect size observed for connectivity between the default mode network and the dorsal attention network (B = 0.0006, t = 5.57, p-FDR < 0.001, partial-r = 0.06, 95% CI = 0.0004, 0.0008). We report significant ADHD-related differences in interactions between the default mode network and task-positive networks, in line with default mode interference models of ADHD. Effect sizes (Cohen’s d and partial-r, estimated from the mega-analytic models) were small, indicating subtle group differences. The overlap between the affected brain networks in the clinical and general population samples supports the notion of brain phenotypes operating along an ADHD continuum.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Association AP. Diagnostic and statistical manual of mental disorders (DSM-5®). American Psychiatric Pub; 2013.
Bozhilova NS, Michelini G, Kuntsi J, Asherson P. Mind wandering perspective on attention-deficit/hyperactivity disorder. Neurosci Biobehav Rev. 2018;92:464–76.
Sonuga-Barke EJS, Castellanos FX. Spontaneous attentional fluctuations in impaired states and pathological conditions: a neurobiological hypothesis. Neurosci Biobehav Rev. 2007;31:977–86.
Castellanos FX, Proal E. Large-scale brain systems in ADHD: beyond the prefrontal–striatal model. Trends Cogn Sci. 2012;16:17–26.
Raichle ME. The brain’s default mode network. Annu Rev Neurosci. 2015;38:433–47.
Thomas Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106:1125–65.
Castellanos FX, Kelly C, Milham MP. The restless brain: attention-deficit hyperactivity disorder, resting—state functional connectivity, and intrasubject variability. Can J Psychiatry. 2009;54:665–72.
Mills BD, Miranda-Dominguez O, Mills KL, Earl E, Cordova M, Painter J, et al. ADHD and attentional control: Impaired segregation of task positive and task negative brain networks. Network. Neuroscience. 2018;2:200–17.
Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL. Functional-anatomic fractionation of the brain’s default network. Neuron. 2010;65:550–62.
Weissman DH, Roberts KC, Visscher KM, Woldorff MG. The neural bases of momentary lapses in attention. Nat Neurosci. 2006;9:971–8.
Christoff K, Gordon AM, Smallwood J, Smith R, Schooler JW. Experience sampling during fMRI reveals default network and executive system contributions to mind wandering. Proc Natl Acad Sci USA. 2009;106:8719–24.
Sudre G, Szekely E, Sharp W, Kasparek S, Shaw P. Multimodal mapping of the brain’s functional connectivity and the adult outcome of attention deficit hyperactivity disorder. Proc Natl Acad Sci. 2017;114:11787–92.
Sripada C, Kessler D, Fang Y, Welsh RC, Prem Kumar K, Angstadt M. Disrupted network architecture of the resting brain in attention‐deficit/hyperactivity disorder. Hum Brain Mapp. 2014;35:4693–705.
Kernbach JM, Satterthwaite TD, Bassett DS, Smallwood J, Margulies D, Krall S, et al. Shared endo-phenotypes of default mode dysfunction in attention deficit/hyperactivity disorder and autism spectrum disorder. Transl Psychiatry. 2018;8:1–11.
Sun L, Cao Q, Long X, Sui M, Cao X, Zhu C, et al. Abnormal functional connectivity between the anterior cingulate and the default mode network in drug-naïve boys with attention deficit hyperactivity disorder. Psychiatry Res: Neuroimaging. 2012;201:120–7.
Lin H-Y, Kessler D, Tseng W-YI, Gau SS-F. Increased functional segregation related to the salience network in unaffected siblings of youths with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2021;60:152–65.
Mostert JC, Shumskaya E, Mennes M, Onnink AMH, Hoogman M, Kan CC, et al. Characterising resting-state functional connectivity in a large sample of adults with ADHD. Prog Neuro-Psychopharmacol Biol Psychiatry. 2016;67:82–91.
Jung M, Tu Y, Park J, Jorgenson K, Lang C, Song W, et al. Surface-based shared and distinct resting functional connectivity in attention-deficit hyperactivity disorder and autism spectrum disorder. Br J Psychiatry. 2019;214:339–44.
Sidlauskaite J, Sonuga-Barke E, Roeyers H, Wiersema JR. Altered intrinsic organisation of brain networks implicated in attentional processes in adult attention-deficit/hyperactivity disorder: a resting-state study of attention, default mode and salience network connectivity. Eur Arch Psychiatry Clin Neurosci. 2016;266:349–57.
Cortese S, Aoki YY, Itahashi T, Castellanos FX, Eickhoff SB. Systematic review and meta-analysis: resting state functional magnetic resonance imaging studies of attention-deficit/hyperactivity disorder. J Am Acad Child Adolescent Psych. 2021;60:61–75.
Button KS, Ioannidis JP, Mokrysz C, Nosek BA, Flint J, Robinson ES, et al. Power failure: why small sample size undermines the reliability of neuroscience. Nat Rev Neurosci. 2013;14:365–76.
Gao Y, Shuai D, Bu X, Hu X, Tang S, Zhang L, et al. Impairments of large-scale functional networks in attention-deficit/hyperactivity disorder: a meta-analysis of resting-state functional connectivity. Psychological Med. 2019;49:2475–85.
Sutcubasi B, Metin B, Kurban MK, Metin ZE, Beser B, Sonuga-Barke E. Resting-state network dysconnectivity in ADHD: A system-neuroscience-based meta-analysis. The. World J Biol Psychiatry. 2020;21:662–72.
Ciric R, Rosen AF, Erus G, Cieslak M, Adebimpe A, Cook PA, et al. Mitigating head motion artifact in functional connectivity MRI. Nat Protoc. 2018;13:2801–26.
Ciric R, Wolf DH, Power JD, Roalf DR, Baum GL, Ruparel K, et al. Benchmarking of participant-level confound regression strategies for the control of motion artifact in studies of functional connectivity. Neuroimage. 2017;154:174–87.
Hoogman M, Bralten J, Hibar DP, Mennes M, Zwiers MP, Schweren L, et al. Subcortical brain volume differences of participants with ADHD across the lifespan: an ENIGMA collaboration. Lancet Psychiatry. 2017;4:310.
Hoogman M, Muetzel R, Guimaraes JP, Shumskaya E, Mennes M, Zwiers MP, et al. Brain imaging of the cortex in ADHD: a coordinated analysis of large-scale clinical and population-based samples. Am J Psychiatry. 2019;176:531–42.
Milham MP, Fair D, Mennes M, Mostofsky SH. The ADHD-200 consortium: a model to advance the translational potential of neuroimaging in clinical neuroscience. Frontiers in Systems. Neuroscience. 2012;6:62.
Alexander LM, Escalera J, Ai L, Andreotti C, Febre K, Mangone A, et al. An open resource for transdiagnostic research in pediatric mental health and learning disorders. Sci Data. 2017;4:1–26.
Casey BJ, Cannonier T, Conley MI, Cohen AO, Barch DM, Heitzeg MM, et al. The adolescent brain cognitive development (ABCD) study: imaging acquisition across 21 sites. Developmental Cogn Neurosci. 2018;32:43–54.
Sudre G, Sharp W, Kundzicz P, Bouyssi-Kobar M, Norman L, Choudhury S, et al. Predicting the course of ADHD symptoms through the integration of childhood genomic, neural, and cognitive features. Mol Psychiatry. 2021;26:4046–54.
Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59:2142–54.
Satterthwaite TD, Wolf DH, Roalf DR, Ruparel K, Erus G, Vandekar S, et al. Linked sex differences in cognition and functional connectivity in youth. Cereb Cortex. 2015;25:2383–94.
Chabernaud C, Mennes M, Kelly C, Nooner K, Di Martino A, Castellanos FX, et al. Dimensional brain-behavior relationships in children with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2012;71:434–42.
Liddle EB, Hollis C, Batty MJ, Groom MJ, Totman JJ, Liotti M, et al. Task‐related default mode network modulation and inhibitory control in ADHD: Effects of motivation and methylphenidate. J Child Psychol Psychiatry. 2011;52:761–71.
Thapar A. Discoveries on the genetics of ADHD in the 21st century: new findings and their implications. Am J Psychiatry. 2018;175:943–50.
Shaw P, Gilliam M, Liverpool M, Weddle C, Malek M, Sharp W, et al. Cortical development in typically developing children with symptoms of hyperactivity and impulsivity: support for a dimensional view of attention deficit hyperactivity disorder. Am J Psychiatry. 2011;168:143–51.
LA, ATR. Manual for the ASEBA School-Age Forms & Profiles. Burlington, VT: University of Vermont. Research Center for Children, Youth, & Families. 2001.
Brown SA, Brumback T, Tomlinson K, Cummins K, Thompson WK, Nagel BJ, et al. The National Consortium on Alcohol and NeuroDevelopment in Adolescence (NCANDA): a multisite study of adolescent development and substance use. J Stud Alcohol Drugs. 2015;76:895–908.
Somerville LH, Bookheimer SY, Buckner RL, Burgess GC, Curtiss SW, Dapretto M, et al. The Lifespan Human Connectome Project in Development: A large-scale study of brain connectivity development in 5–21 year olds. Neuroimage. 2018;183:456–68.
Pfefferbaum A, Rohlfing T, Pohl KM, Lane B, Chu W, Kwon D, et al. Adolescent development of cortical and white matter structure in the NCANDA sample: role of sex, ethnicity, puberty, and alcohol drinking. Cereb Cortex. 2016;26:4101–21.
Müller-Oehring EM, Kwon D, Nagel BJ, Sullivan EV, Chu W, Rohlfing T, et al. Influences of age, sex, and moderate alcohol drinking on the intrinsic functional architecture of adolescent brains. Cereb Cortex. 2018;28:1049–63.
Zhao Q, Sullivan EV, Honnorat N, Adeli E, Podhajsky S, De Bellis MD, et al. Association of heavy drinking with deviant fiber tract development in frontal brain systems in adolescents. JAMA Psychiatry. 2021;78:407–15.
Pohl KM, Sullivan EV, Pfefferbaum A. The NCANDA_PUBLIC_BASE_RESTINGSTATE_V01 Data Release of the National Consortium on Alcohol and NeuroDevelopment in Adolescence (NCANDA). Sage Bionetworks Synapse. 2017;2017.
Pohl KM, Sullivan EV, Pfefferbaum A. The NCANDA_PUBLIC_4Y_REDCAP_V01 Data Release of the National Consortium on Alcohol and NeuroDevelopment in Adolescence (NCANDA). Sage Bionetworks Synapse. 2021;2021.
Pohl KM, Sullivan EV, Pfefferbaum A. The NCANDA_PUBLIC_BASE_STRUCTURAL_V01 Data Release of the National Consortium on Alcohol and NeuroDevelopment in Adolescence (NCANDA). Sage Bionetworks Synapse. 2017;2017.
Sudre G, Bouyssi-Kobar M, Norman L, Sharp W, Choudhury S, Shaw P. Estimating the heritability of developmental change in neural connectivity, and its association with changing symptoms of attention-deficit/hyperactivity disorder. Biol Psychiatry. 2021;89:443–50.
Ho DE, Imai K, King G, Stuart EA. MatchIt: Nonparametric Preprocessing for Parametric Causal Inference. J Stat Softw. 2011;42:1–28.
Esteban O, Markiewicz CJ, Blair RW, Moodie CA, Isik AI, Erramuzpe A, et al. fMRIPrep: a robust preprocessing pipeline for functional MRI. Nat Methods. 2019;16:111–6.
Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. FSL. Neuroimage. 2012;62:782–90.
Cho JW, Korchmaros A, Vogelstein JT, Milham MP, Xu T. Impact of concatenating fMRI data on reliability for functional connectomics. Neuroimage. 2021;226:117549.
Pruim RH, Beckmann CF, Oldehinkel M, Oosterlaan J, Heslenfeld D, Hartman CA, et al. An integrated analysis of neural network correlates of categorical and dimensional models of attention-deficit/hyperactivity disorder. Biol Psychiatry: Cogn Neurosci Neuroimaging. 2019;4:472–83.
Norman LJ, Sudre G, Bouyssi-Kobar M, Sharp W, Shaw P. A longitudinal study of resting-state connectivity and response to psychostimulant treatment in ADHD. Am J Psychiatry. 2021;178:744–51.
Norman LJ, Sudre G, Bouyssi-Kobar M, Sharp W, Shaw P. An examination of the relationships between attention/deficit hyperactivity disorder symptoms and functional connectivity over time. Neuropsychopharmacology. 2022;47:704–10.
Von Rhein D, Oldehinkel M, Beckmann CF, Oosterlaan J, Heslenfeld D, Hartman CA, et al. Aberrant local striatal functional connectivity in attention‐deficit/hyperactivity disorder. J Child Psychol Psychiatry. 2016;57:697–705.
Schaefer A, Kong R, Gordon EM, Laumann TO, Zuo X-N, Holmes AJ, et al. Local-global parcellation of the human cerebral cortex from intrinsic functional connectivity MRI. Cereb Cortex. 2018;28:3095–114.
Kuznetsova A, Brockhoff PB, Christensen RH. lmerTest package: tests in linear mixed effects models. J Stat Softw. 2017;82:1–26.
Yan C-G, Chen X, Li L, Castellanos FX, Bai T-J, Bo Q-J, et al. Reduced default mode network functional connectivity in patients with recurrent major depressive disorder. Proc Natl Acad Sci. 2019;116:9078–83.
Boedhoe PS, Heymans MW, Schmaal L, Abe Y, Alonso P, Ameis SH, et al. An empirical comparison of meta-and mega-analysis with data from the ENIGMA obsessive-compulsive disorder working group. Front Neuroinformatics. 2019;12:102.
Miller GA, Chapman JP. Misunderstanding analysis of covariance. J Abnorm Psychol. 2001;110:40.
Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc: Ser B (Methodol). 1995;57:289–300.
Cordova M, Antovich DM, Ryabinin P, Neighbor C, Mooney MA, Dieckmann N, et al. ADHD: Restricted Phenotypes Prevalence, Comorbidity, and Polygenic Risk Sensitivity in ABCD Baseline Cohort. 2021;2021.
D’Agati E, Curatolo P, Mazzone L. Comorbidity between ADHD and anxiety disorders across the lifespan. Int J Psychiatry Clin Pract. 2019;23:238–44.
Picon FA, Sato JR, Anés M, Vedolin LM, Mazzola AA, Valentini BB, et al. Methylphenidate alters functional connectivity of default mode network in drug-naive male adults with ADHD. J Atten Disord. 2020;24:447–55.
Christakou A, Murphy CM, Chantiluke K, Cubillo AI, Smith AB, Giampietro V, et al. Disorder-specific functional abnormalities during sustained attention in youth with attention deficit hyperactivity disorder (ADHD) and with autism. Mol Psychiatry. 2013;18:236–44.
Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–15.
Uddin LQ, Kelly AC, Biswal BB, Margulies DS, Shehzad Z, Shaw D, et al. Network homogeneity reveals decreased integrity of default-mode network in ADHD. J Neurosci Methods. 2008;169:249–54.
Baum GL, Ciric R, Roalf DR, Betzel RF, Moore TM, Shinohara RT, et al. Modular segregation of structural brain networks supports the development of executive function in youth. Curr Biol. 2017;27:1561–72. e8.
Kelly AC, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. Competition between functional brain networks mediates behavioral variability. Neuroimage. 2008;39:527–37.
Duffy KA, Rosch KS, Nebel MB, Seymour KE, Lindquist MA, Pekar JJ, et al. Increased integration between default mode and task-relevant networks in children with ADHD is associated with impaired response control. Dev Cogn Neurosci. 2021;50:100980.
Godwin CA, Hunter MA, Bezdek MA, Lieberman G, Elkin-Frankston S, Romero VL, et al. Functional connectivity within and between intrinsic brain networks correlates with trait mind wandering. Neuropsychologia. 2017;103:140–53.
Sadaghiani S, Poline J-B, Kleinschmidt A, D’Esposito M. Ongoing dynamics in large-scale functional connectivity predict perception. Proc Natl Acad Sci. 2015;112:8463–8.
Shappell HM, Duffy KA, Rosch KS, Pekar JJ, Mostofsky SH, Lindquist MA, et al. Children with attention-deficit/hyperactivity disorder spend more time in hyperconnected network states and less time in segregated network states as revealed by dynamic connectivity analysis. NeuroImage. 2021;229:117753.
Demontis D, Walters RK, Martin J, Mattheisen M, Als TD, Agerbo E, et al. Discovery of the first genome-wide significant risk loci for attention deficit/hyperactivity disorder. Nat Genet. 2019;51:63–75.
Pardoe HR, Hiess RK, Kuzniecky R. Motion and morphometry in clinical and nonclinical populations. Neuroimage. 2016;135:177–85.
Marek S, Tervo-Clemmens B, Calabro FJ, Montez DF, Kay BP, Hatoum AS, et al. Reproducible brain-wide association studies require thousands of individuals. Nature. 2022;603:654–60.
Karcher NR, Michelini G, Kotov R, Barch DM. Associations between resting-state functional connectivity and a hierarchical dimensional structure of psychopathology in middle childhood. Biol Psychiatry: Cogn Neurosci Neuroimaging. 2021;6:508–17.
Norman LJ, Taylor SF, Liu Y, Radua J, Chye Y, De Wit SJ, et al. Error processing and inhibitory control in obsessive-compulsive disorder: a meta-analysis using statistical parametric maps. Biol Psychiatry. 2019;85:713–25.
Bernanke J, Luna A, Chang L, Bruno E, Dworkin J, Posner J. Structural brain measures among children with and without ADHD in the Adolescent Brain and Cognitive Development Study cohort: a cross-sectional US population-based study. Lancet Psychiatry. 2022;9:222–31.
Mooney MA, Hermosillo RJ, Feczko E, Miranda-Dominguez O, Moore LA, Perrone A, et al. Cumulative Effects of Resting-state Connectivity Across All Brain Networks Significantly Correlate with ADHD Symptoms. MedRxiv. 2021;2021.
Shen X, Finn ES, Scheinost D, Rosenberg MD, Chun MM, Papademetris X, et al. Using connectome-based predictive modeling to predict individual behavior from brain connectivity. Nat Protoc. 2017;12:506–18.
Wolfers T, Beckmann CF, Hoogman M, Buitelaar JK, Franke B, Marquand AF. Individual differences v. the average patient: mapping the heterogeneity in ADHD using normative models. Psychological Med. 2020;50:314–23.
Scheinost D, Noble S, Horien C, Greene AS, Lake EM, Salehi M, et al. Ten simple rules for predictive modeling of individual differences in neuroimaging. NeuroImage. 2019;193:35–45.
Sripada C, Rutherford S, Angstadt M, Thompson WK, Luciana M, Weigard A, et al. Prediction of neurocognition in youth from resting state fMRI. Mol Psychiatry. 2020;25:3413–21.
Sripada C, Angstadt M, Rutherford S, Kessler D, Kim Y, Yee M, et al. Basic units of inter-individual variation in resting state connectomes. Sci Rep. 2019;9:1–12.
Woo C-W, Chang LJ, Lindquist MA, Wager TD. Building better biomarkers: brain models in translational neuroimaging. Nat Neurosci. 2017;20:365–77.
Shaw P, Eckstrand K, Sharp W, Blumenthal J, Lerch JP, Greenstein D, et al. Attention-deficit/hyperactivity disorder is characterized by a delay in cortical maturation. Proc Natl Acad Sci. 2007;104:19649–54.
Dafflon J, Da Costa PF, Váša F, Monti RP, Bzdok D, Hellyer PJ, et al. Neuroimaging: into the Multiverse. BioRxiv. 2020;2020.
Couvy-Duchesne B, Ebejer JL, Gillespie NA, Duffy DL, Hickie IB, Thompson PM, et al. Head motion and inattention/hyperactivity share common genetic influences: implications for fMRI studies of ADHD. PloS One. 2016;11:e0146271.
Acknowledgements
Data used in the preparation of this article were obtained from the Adolescent Brain Cognitive Development (ABCD) Study (https://abcdstudy.org), held in the NIMH Data Archive (NDA). This is a multisite, longitudinal study designed to recruit more than 10,000 children aged 9 to 10 years and follow them over 10 years into early adulthood. The ABCD Study is supported by the National Institutes of Health and additional federal partners under award numbers U01DA041048, U01DA050989, U01DA051016, U01DA041022, U01DA051018, U01DA051037, U01DA050987, U01DA041174, U01DA041106, U01DA041117, U01DA041028, U01DA041134, U01DA050988, U01DA051039, U01DA041156, U01DA041025, U01DA041120, U01DA051038, U01DA041148, U01DA041093, U01DA041089, U24DA041123, and U24DA041147. A full list of supporters is available at https://abcdstudy.org/ federal-partners.html. A listing of participating sites and a complete listing of the study investigators can be found at https://abcdstudy.org/consortium_ members/. ABCD consortium investigators designed and implemented the study and/or provided data but did not necessarily participate in analysis or writing of this report. The ABCD data repository grows and changes over time. The ABCD data used in this report came from DOI: 10.15154/1519007. The DOIs can be found at https://doi.org/10.15154/1519007 and from the fast-track data release (raw neuroimaging data). The raw data are available at https://nda.nih.gov/edit_collection.html?id=2573. Instructions on how to create an NDA study are available at https://nda.nih.gov/training/modules/study.html). Data and/or research tools used in the preparation of this manuscript were obtained from the National Institute of Mental Health (NIMH) Data Archive (NDA). NDA is a collaborative informatics system created by the National Institutes of Health to provide a national resource to support and accelerate research in mental health. Dataset identifier (s): #2573, #2846 and #3165. This manuscript reflects the views of the authors and may not reflect the opinions or views of the NIH or of the Submitters submitting original data to NDA (https://doi.org/10.15154/1527788). The Human Connectome Project-Development was supported by the NIMH under Award Number U01MH109589. This manuscript included data from a limited access dataset obtained from the Child Mind Institute Biobank, the HBN study (http://www.healthybrainnetwork.org). Data collection as for the ADHD200-NYU cohort was funded by the NIMH (R01MH083246), Autism Speaks, The Stavros Niarchos Foundation, The Leon Levy Foundation, and an endowment provided by Phyllis Green and Randolph Cōwen. Data collection for the ADHD200-Peking cohort was supported by The Commonwealth Sciences Foundation, Ministry of Health, China (200802073), The National Foundation, Ministry of Science and Technology, China (2007BAI17B03), The National Natural Sciences Foundation, China (30970802), The Funds for International Cooperation of the National Natural Science Foundation of China (81020108022), The National Natural Science Foundation of China (8100059), Open Research Fund of the State Key Laboratory of Cognitive Neuroscience and Learning. Data collection and sharing for this project was provided by the National Consortium on Alcohol and Neurodevelopment in Adolescence (NCANDA), which is funded by the U.S. National Institute on Alcohol Abuse and Alcoholism with co-funding from the National Institute on Drug Abuse, the National Institute of Mental Health, the National Institute of Child Health and Human Development, and the National Institute of Health Office of the Director (AA021695 (MPI: SA Brown, SF Tapert), AA021697 (MPI: A Pfefferbaum, KM Pohl), AA021692 (PI: SF Tapert), AA021681 (PI: MD DeBellis), AA021690 (PI: DB Clark), AA021691 (PI: B Nagel), AA021696 (MPI: IM Colrain, FC Baker), AA021697-04S1 (PI: KM Pohl)). NCANDA data are disseminated by the Center for Health Sciences, SRI International. We used data from NCANDA_PUBLIC_4Y_REDCAP_V01 (https://doi.org/10.7303/syn22216455), NCANDA_PUBLIC_BASE_RESTINGSTATE_V01 (https://doi.org/10.7303/syn11605291) and NCANDA_PUBLIC_BASE_STRUCTURAL_V01 (https://doi.org/10.7303/syn11541569). This study reflects the views of the authors and may not reflect the opinions or views of other individuals or institutions including the NIH, the ABCD, NCANDA, HCP or ADHD200 consortium investigators, the Child Mind Institute, SRI International, or other funding agencies. Image processing was conducted using the high-performance computing capabilities of the NIH Biowulf cluster. The authors thank the NIMH Data Science and Sharing Team for help with accessing and processing the ABCD-BIDS dataset.
Funding
Funded by the intramural research program of the National Institute of Mental Health and the National Human Genome Research Institute (ZIAHG200378 to PS). This funding supported data collection for the NCR cohort (ClinicalTrials.gov identifier: NCT01721720).
Author information
Authors and Affiliations
Contributions
Study concept and design: LJN. Acquisition, analysis, or interpretation of data: all authors. Drafting of the manuscript: LJN, PS. Critical revision of the manuscript for important intellectual content: All authors. Statistical analysis: LJN, GS. Administrative, technical, or material support: LJN, GS, PS. Study supervision: PS. Obtained funding: PS.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Norman, L.J., Sudre, G., Price, J. et al. Evidence from “big data” for the default-mode hypothesis of ADHD: a mega-analysis of multiple large samples. Neuropsychopharmacol. 48, 281–289 (2023). https://doi.org/10.1038/s41386-022-01408-z
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41386-022-01408-z
This article is cited by
-
External trigeminal nerve stimulation in youth with ADHD: a randomized, sham-controlled, phase 2b trial
Nature Medicine (2026)
-
Enhanced individual difference of functional brain network induced by volitional eyes closing
Scientific Reports (2025)
-
Cross-sectional mega-analysis of resting-state alterations associated with autism and attention-deficit/hyperactivity disorder in children and adolescents
Nature Mental Health (2025)
-
Harnessing mega-analysis in the era of “big data” neuroimaging
Neuropsychopharmacology (2025)
-
Multiscale functional connectivity reveals imbalanced interplay between higher- and lower-order brain networks in ADHD
Nature Mental Health (2025)